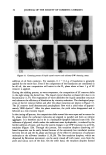
LIQUID CRYSTAL MAKE-UP REMOVER 31 CLEANSING MECHANISM AND CHARACTERISTICS OF LIQUID CRYSTAL MAKE-UP REMOVER The cleansing ability of different surfactant systems and the droplet diameter of emul- sions that formed during the rinse-off process are compared in Figure 10. The cleansing ability depended upon the droplet diameter of the emulsions, and only the liquid crystal make-up remover (LC remover), which formed a fine submicroemulsion, completely removed the model oily cosmetics. The cleansing processes of the LC remover and ordinary O/W cleansing cream are shown in Figure 11. The LC remover was applied on the marked hand. Though both make-up removers dissolved and dispersed the model oily cosmetics, only the LC remover could remove it almost completely merely by spraying with water. The cleansing mechanism of LC remover is described in terms of phase state in a triangle phase diagram in Figure 12. In order to indicate phase transitions during the rubbing and rinsing-off processes, the water component is drawn at an apex of the diagram. The composition of LC remover presented in Table I, marked with •r on the diagram, is identified as a lameliar liquid crystalline phase (D 2 phase) (Figure 12). In actual usage, the composition of the LC remover is supposed to shift toward the oil apex owing to the lOO 9o 8o 7o o.1 EOD-20 ESMS-20 I ß I EHCO-60 I I EMGS-15 I - I EO-20 , I • I • I 1 10 100 Droplet diameter (pm) Figure 10. Cleansing ability of different surfactant systems and droplet diameters of emulsion formed during rinsing-off process,
32 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) (3) (4) Figure 11. Cleansing process of liquid crystal remover and ordinary O/W cleansing cream. addition of oil from cosmetics. For example, 0.1 -- 0.4 g of foundation is generally applied for the entire face. Even if the components of the foundation are considered to be all oil, the new composition still exists in the D 2 phase when at least 1 g of LC remover is applied. During the rubbing process, as water evaporates, the composition of LC remover shifts to the right along the dotted line. The liquid crystal dissolves oil-based dirt due to its bicontinuity (1,17). The evaporation of water abolishes the liquid crystalline structure and enhances the efficiency of dissolution by viscosity reduction. The rheological prop- erties of the LC remover before and after the phase transition are shown in Figure 13. The LC remover itself demonstrated pseudoplastic flow with a yield value of approxi- mately 5000 dyrdcm 2. After the phase transition, the yield value disappeared and it behaved as a low-viscosity liquid. In the rinsing-off process, the composition shifts toward the water apex and re-enters the D 2 phase where the surfactant molecules are aligned in parallel and form an infinite aggregate. It is therefore said to be in a hydrophile-lipophile balanced state (18). The influence of glycerol, which makes the surfactant more hydrophobic, is reduced by the further addition of water, and thus the system changes to an O/W emulsion via an OlD 2 emulsion (Figure 12b). Through this process, fine emulsion droplets dissolving oil- based impurities can be easily formed because of the extremely low interfacial tension between the oil and the D 2 phase and because of the effective orientation of surfactant molecules at the oil/water interface (1,19,20). The fine emulsion droplets, which are dispersed by Brownian movement, can be easily rinsed off with water, even from the pores and sulcus cutis of the skin. On the other hand, the emulsion droplets formed
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)