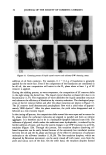
RHEOLOGIC MEASUREMENTS 10 1 .1 o .01 .01 [] [] [] [] [] ß ß O [] ß ß ß ß 0 ß 0 ß ß ß .1 1 10 100 (b (Rad/s) Figure 2. Effect of xanthan gum concentration on storage and loss moduli (G' and G", respectively). C), G' 0.2% O, G" 0.2% [•, G' 0.4%' I, G" 0.4%. (Figure 5). The data for MAS are representative of rigid structures in which viscosity increases toward infinity as the shear stress is decreased. This is the definition of yield value. XG at a concentration of 0.4%, on the other hand, behaves more like a structured fluid system at very low shear, shear stress and shear rate are proportional so that apparent viscosity is constant. There is thus no evidence for a yield value for the XG solution, in agreement with previous work (2,7). The shear rates for the data in Figure 5 can be calculated as the ratio of shear stress to apparent viscosity shear rate values on the high-viscosity end of the curve (left side) for XG are of the order of 10-4 s-• Apparently such low values are necessary to demonstrate the existence of a low-shear constant-viscosity region for this gum. The literature contains contradictory statements regarding whether or not XG has a yield value. Part of the problem is that yield value can be estimated in a variety of ways not all of which are equivalent. It is sometimes inferred from a flow curve based on data obtained at relatively high shear rates. In such cases, extrapolation to the shear stress axis might be used to determine a "practical" yield value, which may empirically correlate with macroscopic observations of dispersion stability though it does not correspond to an actual yield value. Earlier work in this laboratory utilizing a continuous shearing procedure did not indicate the existence of a yield value, but did suggest that xanthan at moderate concentrations (0.3% and higher) was in a "gel-like" state when undis- turbed (1). The ability of XG to retard settling of dispersed particles is well known. Sedimentation
6 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS lO ß [] [] ß [] ß ß o o o $ o [] $ o ß [] [] $ $ o ß ß [] ß .Ol .1 1 lO lOO to (Rad/s) Figure •. Foeoe½½• ooex•n•h•n •um ½on•½n•don on 1o$$ •n•½n•. ¸, 0. [%' •, 0.P%' I•, 0.•%' •, 0.4%. rates of suspensions and emulsions were highly dependent on XG concentration in many cases, sedimentation in dispersions containing 0.4% XG was negligible during several months (8,9). Based on our results and data from previous studies, we attribute XG effectiveness in reducing sedimentation to a gel-like but not completely rigid structure and high apparent viscosity at low shear, approximately 200 Pas (or 200,000 cp) in the case of 0.4% XG. Values of G' and G" and the loss tangent for MAS-XG combinations are compared to those for MAS alone in Figures 6 through ! 1. The addition of XG to MAS raises G" values (Figures 6 and 7), and the magnitude of the change is directly related to XG concentration. The straightforward conclusion is that XG raises the viscous contribution in mixtures with MAS. The effect of XG on G' is more complex. The first thing to notice is that the G' curves for the mixtures are not horizontal, in contrast to those for MAS alone (Figures 8 and 9). This suggests a change in structural behavior that may be interpreted as a loss of rigidity. Except for the 0.4% concentration, the addition of XG to 1% MAS lowers the value of G' at low frequencies but has the opposite effect at high frequencies (Figure 8). XG at a 0.4% concentration raises G' of 1% MAS at all frequencies within the attain- able measurement range. With 3% MAS, there is a lowering of G' at low frequency at all XG concentrations, and the effect is not strongly concentration-dependent (Figure 9). However, as the frequency is raised, the value of G' for the mixtures increases with XG concentration.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)