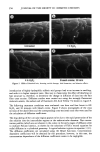
ETHOXYLATION OF HYDROXY ACIDS 323 12 10 --0.15 KOH .30 KOH No Catalyst O.6 KOH Reaction Condition 1. Temperature 150 C 2. Initial Pressure 45 3. Catalyst level varied 0 0.5 1 1.5 2 2.5 3 3.5 Time At Reaction Condition (•n. .... ) Figure 3. Stearic acid ethoxylation rates. Published data indicate that the ratios of mono- to di- ester are as follows (11): Hydrophobe Moles EO Mono ester Diester PEGS* Stearic acid 9.0 38.9 41. ! 20.0 Oleic acid ! 3.6 59.7 26.8 ! 3.5 As earlier stated, the salient difference between fatty acid ethoxylation and fatty alcohol ethoxylation under base catalysis is that the former has an induction period. During this early stage of the reaction, there is a period of time in which there are negligible amounts of product formed. After that initial induction period, the reaction rate in- creases to about that of the fatty alcohol. As Figure 3 shows, increasing the amount of alkaline catalyst simply shortens the induction period but does not eliminate it. The ethoxylation of fatty acids, like that of fatty alcohols, will not occur without a catalyst. The catalyst can be either acidic or, more typically, alkaline. Alkaline catalysts generally produce fewer by-products. EXPERIMENTAL HYDROGENATED CASTOR ETHOXYLATION--ETHENIFICATION REACTION Hydrogenated castor oil is principally 12-hydroxystearic triglyceride. Hydrogenated castor ethoxylates are items of commerce and are commonly used in several applications, including textile and personal care applications. It was originally thought that these materials ethoxylated almost exclusively on the hydroxyl group. It is now under- * PEGS are polyoxyethylene glycol.
324 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS stood that this material ethoxylates in an unusual manner. The process called etheni- fication is well known and is outlined in equation 1. The ethylene oxide is added both to the ester group and to the hydroxyl group. It is therefore possible to evaluate the relative amounts of ethylene oxide that adds to each functionality. In short, there is a group selectivity or preference for ethoxylation. This group selectivity can be classified as strong, intermediate, or weak, depending on the results obtained with the ethoxylation. Equation 1 (idealized) OH CH3-(CH2)5-CH-(CH2)lo-C(O)-O-CH2 OH CH3-(CH2) 5-CH-(CH2)•o -C(O)-O-CH OH I CH3-(CH2) 5-CH-(CH2)10-C(O)-O-CH2 // + n CH 2 O -- CH 2 (OCH2CH2)a-OH CH3-(CH2)5-CH-(CH2)lo-C(O) (OCH2CH2)x-O-CH 2 (OCH2CH2)b-OH I CH3-(CH2)5-CH-(CH2)lo-C(O) (OCH2CH2)Y-O-CH (OCH2CH2)c-OH I CH3-(CH2)5-CH-(CH2)10-C(O) (OCH2CH2)z-O-CH 2 n=a+b+c+x+y+z The material to be ethoxylated is introduced into a clean dry vessel with the desired catalyst, if any. When all the ethylene oxide has been added, the molar ration of ethylene oxide to fatty material is one to one. The contents are then heated to 150øC under agitation. Vacuum is applied to 20 mm for one hour, to remove any water. The vacuum is released, and ethylene oxide is applied under pressure. The contents are then ethoxylated at 150øC and 45 psig. After all the oxide has been added, the reaction mass is held for one hour at 150øC.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)