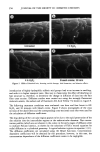
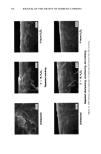
358 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS W (mNIm) '130 11o 90 70 50 ...... Bleach Untr 1/2h 1 h 4h 2'X10 creme Treatment Figure 7. Comparison of average water wettabilities for bleached hair. NaO-• c•O• • 0 COONa Uranin Na salt of fluorescein Figure 8. Molecular structure of uranin used in dye diffusion studies. matrix and increasing fiber swelling, or most likely to a combination of these two effects. Amino acid analysis. Cysteic acid is the major product of cystine oxidation during the bleaching of hair, and so changes in cysteic acid content have been widely studied and reported in the literature. Usually, unbleached hair contains 25-40 Ixmoles/g of cysteic acid. When the hair is bleached, the cysteic acid content increases to 200-400 Ixmoles/ g, and in severely bleached hair, up to 650 Ixmoles/g have been reported (12). A decrease of around 20% in tyrosine and methionine and of 10-15% in lysine and histidine has been reported by Robbins and Kelly (2). The results of the amino acid analysis of the untreated and bleached hair reported in
EVALUATION OF HAIR DAMAGE 359 Table III Diffusion Coefficients of Uranin in Hair Fibers Hair sample D(m2/s X 10- •5) Untreated 1 hour (6% H202) 4 hours (6% 0.5 hours (Bleach creme) 3.59 - 0.67* 9.78 - 3.67 10.30 - 0.87 45.21 - 28.66 * 95% confidence limits. Table IV are limited to cysteic acid formation. In evaluating results, it is important to note the relatively low amount of fiber dissolved during acid hydrolysis for some of the samples. The cysteic acid values given in Table IV are based on the dissolved fraction of the fiber. Expressing the amino acid content in terms of mol % assumes that the undissolved keratin (not measured) has the same composition as the dissolved part, which is rather unlikely. In the case of hair bleached two minutes, ten times in 6% H20 2, only about half the fiber was dissolved and available for assay. For the other samples, dissolution was around 70%, which is considered "normal." The most damaged sample, according to this technique of analysis, is the one bleached for four hours in 6% H202, which produced a level of cysteic acid of "•300 •xmoles/g. A lower level of damage, in the range of 192 and 211 •xmoles/g is found for the samples treated one hour in 6% H202 and with the bleach creme, respectively. All three results fall into the range of expected values for bleached hair, between 200 and 400 •xmoles/g, although the results may be affected by the low percentage of dissolved fiber. The cysteic acid levels achieved in this series of bleached hair is considerably below that for severely bleached or frosted hair (--650 Ixmoles/g) (12). A decrease in tyrosine or methionine was not observed. The analysis did, however, detect a direct time dependence of damage in the series of hair samples bleached with 6% H202. Mechanical properties 1. Bleached hair. The disruption of cystine crosslinks due to bleaching has a major influence on the wet tensile properties of hair. It is well established that in both hair and wool the disulfide bonds contribute largely to wet strength, while the dry strength of the fibers remains unaffected unless more than 60% of the cystine crosslinks have been broken (2). Dry mechanical properties appear to be more sensitive to peptide bond breakdown. A summary of the effects of oxidative treatments on mechanical properties is given in Table V. The mechanical properties fall into two groups. The ultimate properties, involving measurements at the breaking point of the fibers, show significant differences from the untreated level only after the bleach creme treatment. This suggests that the other treatments cause only minor changes in these properties. However, the evidence obtained from the previously discussed analyses of structural change (dye diffusion and AA analysis) indicates that the 6% H20 2 treatments also cause considerable structural damage, suggesting that the trends shown in Table VI have some validity. The non- ultimate mechanical properties (initial modulus, yield stress, and 20% work) show that the four hour, 6% H20 2 treatment is indeed damaging, suggesting that mechanical properties that are not measured at the point of fiber failure are more sensitive to oxidative damage.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)