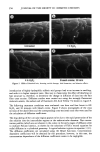
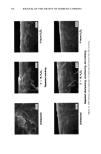
j. Soc. Cosmet. Chem., 44, 337-345 (November/December 1993) Consistency development and destabilization of a model cream LORRAINE E. PENA, BARBARA L. LEE, and JAMES F. STEARNS, Drug Delivery R&D--Specialty Products, The Upjohn Company, Kalamazoo, MI 49007. Received.July 22, 1992. Presented at the 16th IFSCC Congress, New York, October 1990. Synopsis Emulsions are thermodynamically unstable and with time show progressive signs of this instability with eventual phase separation. It is also an established fact that creams based on nonionic emulsifier systems exhibit an initial period of delayed consistency development prior to the destabilization process. To illustrate these phenomena, a cream having a nonionic emulsifier system is destabilized by a surface-active ingredient that also exaggerates the period of retarded consistency development. Using rheologic and microscopic techniques, this study presents a systematic approach by which consistency development and destabilization can be monitored. Characteristic patterns in the rheograms upon aging are correlated with changes seen microscopically, and specific changes signaling the beginning of the destabilization process are identified. Rheologically, destabilization becomes apparent through formation of additional spurs and inflections at low shear rates, a decrease in hysteresis, and a shift to lower maximum shear stress values. Microscopically, polarized light shows formation of diffuse, weakly birefringent structures while ordinary light shows an increase in droplet size. Thermal optical videomicroscopy and trace substance analysis have identified the structures as segments of agglomerated oil phase and verify the photomicroscopy observation that the oil and wax components phase separate as distinct entities. INTRODUCTION All emulsion systems undergo a process of alestabilization and phase separation. How- ever, creams based on nonionic emulsifier systems first undergo an initial period in which their consistency slowly develops. The gel network theory has shown that the consistency of a cream is due to structuring of the continuous phase via penetration and swelling of the fatty amphiphile component by the aqueous surfactant phase to form a viscoelastic, lamellar liquid crystalline gel network (1-6). In the case of nonionic surfactants, this penetration is delayed, and therefore consistency development is also delayed (3). This paper presents a systematic approach by which consistency develop- ment and alestabilization can be monitored. A cetearyl alcohol/ceteareth-20 cream is alestabilized by the addition of a surface-active ingredient that also exaggerates the period of delayed consistency development. Rhe- ology and microscopy are used to monitor consistency development and alestabilization 337
338 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS of the cream. Thermal optical videomicroscopy and trace substance analysis are used to characterize the cream and to identify the composition of the structures observed in the photomicrographs. EXPERIMENTAL FORMULA The cream is based on a commercially available blend of cetearyl alcohol and ceteareth- 20 in a 75/25% (w/w) ratio. The ceteareth-20 is the surfactant that penetrates the fatty amphiphile, cetearyl alcohol, to provide body to the cream in accordance with the gel network theory (1-6). Cetyl palmitate is a wax used to assist in adding body to the cream via formation of a secondary network structure (7). Isopropyl myristate is the liquid internal phase. The identity of the destabilizing ingredient is proprietary, but it can be described as an anionic surfactant. The cream was manufactured using a variable speed mixer with a marine propeller. Emulsification was via the water-to-oil phase inversion technique. A water ring was used to force cool the cream to room temperature. RHEOLOGY AND MICROSCOPY A Ferranti-Shirley cone and plate viscometer was used to determine the rheology of the cream. Samples were tested at 25øC with the instrument in low gear using the 7-cm truncated cone, a 60-sec sweep time, and the 1x scale expansion on the recorder. A shear rate range of 0-164 sec- • was achieved with these instrument settings. Photo- micrographs were obtained with a Zeiss Universal microscope at 500x magnification using both ordinary and polarized light on the same sample. THERMAL OPTICAL VIDEOMICROSCOPY Thermal optical videomicroscopy was performed using polarized light and a 250x magnification. The melting behavior of the cream and its raw materials was studied using a Mettier FP 82 hot stage equipped with photomonitor at a heating rate of 5ø/minute over the range of 25øC to slightly above the melting point. Samples were also studied by videotaping the melting transition. The melting point was defined as the temperature at which a thermal arrest appears in the photomonitor recording and/or the temperature at which the cream or crystals liquefied and flow began. In order to obtain a thin film suitable for microscopy, raw materials were premelted on the slide prior to monitoring their behavior. Cream samples were monitored directly from the initial slide preparation since a thin film was easily produced and because preliminary heating destroyed the sample. RESULTS AND DISCUSSION CONSISTENCY DEVELOPMENT The model cream was a white lotion at the time of manufacture, but it slowly developed
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)