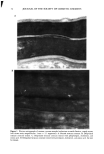
LACTIC ACID STINGING TESTS 11 manufacturer had to recall the product after marketing because of disagreeable reactions. It was then learned that the product was designed for the eye area. It is well known that the thinner eyelids are more susceptible to irritation. Eye area products should therefore be tested directly on that area. Products designed for a specific area of the face should be tested directly on that area. REFERENCES (1) P. J. Frosch and A. M. Kligman, A method for appraising the stinging capacity of topically applied substances, J. Soc. Cosmet. Chem., 28, 197 (1977). (2) A. Fisher, Cosmetic actions and reactions: Therapeutic, irritant and allergic, Cutis, 26, 22 (1980). (3) A. W. Johnson and D. J. Page, Making sense of sensitive skin. Presented at the IFSCC, Yokohama (poster), 1992. (4) B. G. Green and B. S. Bluth, Measuring the chemosensory irritability of human skin,J. Toxicol. Cut. Ocul. Toxicol. 14, 230 (1995). (5) P. J. Frosch and A.M. Kligman, "Recognition of Chemically Vulnerable and Delicate Skin," in Principles of Cosmetics for the Dermatologist, P. J. Frost, Ed. (Mosby, St. Louis, 1981). (6) D. Soschin and A. M. Kligman, "Adverse Subjective Responses," in Safety & Efficacy of Topical Drugs and Cosmetics, A. M. Kligman and J. J. Leyden, Eds. (Grune & Stratton, New York, 1982). (7) D. R. Armstrong, M. L. Dry, C. A. Keele, and J. W. Markham, Methods for studying chemical excitants of pain in man, J. Physiol, 115, 59 (1951). (8) K. Lammintausta, H. I. Maibach, and D. Wilson, Mechanisms of subjective irritation, Dermatosen, 36, 45 (1988). (9) G. Grove, D. Soschin, and A. M. Kligman, Guidelines for performing facial stinging tests, Proc. 12th Congress Internat. Fed. Soc. of Cosmet. Chem, Paris, September 13-17, 1982. (10) J. R. Mayne, O. H. Mills, and J. C. Lyssikatos, Lactic acid sting assay: Reproducibility and sym- metry of subjective sting response. J. Derm. C/in. Eva/. Soc., 3, 63 (1992). (11) P. J. Frosch, "Cutaneous Irritation," in Textbook of Contact Dermatitis, R. J. G. Rycroft, T. Menne, and P. J. Frosch, Eds. (Spring-Verlag, New York, 1995), pp. 28-61. (12) A. Pagnoni, A. M. Kligman, S. el Gainreal, C. Popp, and T. Stoudemayer, An improved procedure for quantitative analysis of sebum production using Sebutape, J. Soc. Cosmet. Chem, 45, 221 (1994). (13) N. Muizzuddin, K. D. Marenus, C. Ethenakis, and D. Maes, Skin reactivity and barrier condition. Presented at the American Academy of Dermatology, Washington, DC, December 4-9, 1993.
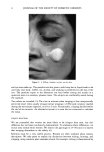
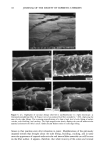
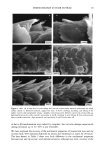
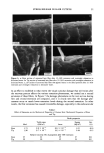
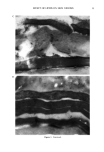
j. Soc. Cosmet. Chem., 47, 13-26 (January/February 1996) Mechanism of tensile stress release in the keratin fiber cuticle: I SIGRID B. RUETSCH and HANS-DIETRICH WEIGMANN, TRI/Princeton, PO Box 625, Princeton NJ 08542. Received September 15, 1995. Synopsis During the extension of keratin fibers, their two major morphological components, the cuticula and the cortex, accommodate the stresses imposed on the fiber each in a totally different fashion. While the latter extends by mechanisms that have been discussed extensively and appear to be well understood, the cuticle cells are essentially inextensible and have to move relative to one another. In the multilayer structure of the cuticular sheath of human hair fibers, this relative movement has to be accommodated by the various layers within each cuticle cell and by the bonding layers between cells, and finally causes the lifting of surface scale edges at higher strain levels. It is proposed that extension mainly causes shear stresses between layers of different composition and extensibility within the cuticle cell. This leads to failure in the weak endocu- ticular layer and results in "delamination" and lifting of the outer layers of the surface cuticle. The damage is irreversible upon release of the fiber and immersion in water, as reflected in the onset of scale lifting at considerably lower strain levels during a second extension. Scale lifting was not observed during the extension of wool fibers, which appears to be a reflection of the higher rigidity of the cuticle cells of wool. INTRODUCTION Standard grooming practices, such as shampooing, combing, and brushing, can cause considerable damage to the cuticle of human hair, as summarized recently by C. R. Robbins (1). Apart from the general abrasive loss of cuticle layers, which can be quite severe and can eventually lead to the complete loss of the cuticular sheath and formation of split ends (2-5), there are various processes that damage the cuticular structure in such a way that scale edges become particularly vulnerable to abrasive action. One of the processes of damage introduction is the imposition of stresses on individual hair fibers during combing, especially when encountering a snag. These stresses result in reversible fiber extension as well as in some irreversible processes involving the cuticle. The two major morphological components of the hair fiber respond quite differently to the stresses introduced into the fiber during extensions beyond the yield point. The cortex is able to release stresses by the unfolding of o• helical structures into the pleated sheet arrangement of the [3 structure. This o•-[3 transformation occurs in the microfibrils that are embedded in and interconnected to the disulfide cross-linked noncrystalline matrix material that deforms along with the unfolding of the o• helices. There is thought 13
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)