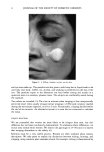
DISULFIDE BOND REDUCTION IN HAIR 57 First Nucleophilic Substitution: Ker-S-S-Ker + HO2C-CH2-SA-H + HO2C-CH2-SB-SB-CH2-CO2H kerabn th•oi disuifide excess A TG Dithiodtglycoltc acid (A) Ker-S-S^-CHa-COaH + Ker-S-H + HOaC-CHa-SB-SB-CHa-COaH m•xed d•sulfide reduced keratin disulfide product (similar to Eq. la) (B) Ker-S-S-Ker + HOaC-CHa-SB-H + HOaC-CHa-S^-SB-CHa-COaH keratin thiol disulfide (monomer-dimer interchange) Second Nucleo@hilic Substitution: (A) Ker-S- S^-CH2-CO2H + HO2C-CH2-S^-H + HO2C-CH2-SB-SB-CH2-CO2H m•xed d•sulfide tNol d•sulfide excess A TG Dithtooyycol•c acid (A•) Ker-S-H + HO2C-CH2-SB-SB-CH2-CO2H reduced keratm d•sulfide product + HO2C-CH2-SA-SA-CH2-CO2H disulfide formed from reaction w•th m•xed d•sulfide (similar to Eq. lb) (A2) Ker-S-SA-CH2-CO2H + HO2C-CH2-SB-H + HO2C-CH2-S^-SB-CH2-CO2H m•xed disulfide th•ol disulfide (monomer-dimer interchange) (B) Ker-S-S-Ker + HO2C-CH2-SA-H + HO2C-CH2-SB-SB-CH2-CO2 H • keratln th•01 d•sulfide excess A TG D•th•odtglycolic acld (B•) Ker-S- SA-CH2-CO2H + HO2C-CH2-SB-SB-CH2-CO2H + Ker-S-H (same as A) mixed d•sulfide dlsulfide reduced keratm (B2) Ker-S-S-Ker + HO2C-CH2-Sa-H + HO2C-CH2-SA-Sa-CH2-CO2H keratin thiol dlsulfide (monomer-dimer interchange) Scheme l. However, hair fibers that were reduced in the presence of DTDG for 5 or 10 minutes were stronger after neutralization, as determined by the 20% index (Table III). No significant difference was found for hair fibers neutralized after 15 minutes of reduction in the presence of DTDG versus reduction in ATG alone. This effect of DTDG on fiber strength following neutralization may be due to a higher formation of mixed disulfide during the reduction step (Ker-S-S-CH2-COOH), which may be more easily converted to native keratin (Ker-S-S-Ker) rather than to cysteic acid or lanthionine. CONCLUSIONS 1. The addition of increasing amounts of exogenous disulfide may reduce overprocessing
58 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS by limiting the extent of reduction of the disulfide bonds via a competitive reaction. However, the kinetics of stress relaxation were not affected when the fiber is held under 1.5 % constant strain, because all three treatments exhibited pseudo first-order kinetics and produced similar reaction rate constants. 2. The addition of dithiodiglycolic acid did not significantly alter the chemical kinetics of stress relaxation or diminish the extent of fiber weakening prior to neutralization as indicated by the 20% index ratio. However, hair reduced in the presence of dithiodi- glycolic acid was stronger after neutralization. REFERENCES (1) R. R. Wickett, Kinetic studies of hair reduction using a single fiber technique,J. Soc. Cosmet. Chem., 34, 301 (1983). (2) R. R. Wickett and B. G. Barman, Factors affecting the kinetics of disulfide bond reduction in hair, J. Soc. Cosmet. Chem., 36, 75 (1985). (3) R. R. Wickett, Disulfide bond reduction in permanent waving, Cosmet. Toilerr., 106, 37 (1991). (4) C. E. Reese and H. Eyring, Mechanical properties and structure of hair, Text. Resh. J., 20, 743 (1950). (5) D. Weigmann, L. Rebenfeld, and C. Dansizer, The role of sulfhydryl groups on the mechanism of permanent setting of wool, Cirtd (Paris), 319-328 (1965). (6) D. Weigmann, L. Rebenfeld, and C. Dansizer, Kinetics and temperature dependence of the chemical stress relaxation of wool fibers, Text. Resh. J., 36, 535 (1966). (7) D. Wiegmann and L. Rebenfeld, The Chemistry ofSulfides, 1st ed. (Interscience Publishers, New York, 1968), pp. 185-203. (8) D. Weigmann and L. Rebenfeld, The reduction of wool with dithiothreitol, Text. Resh. J., 36, 202 (1966). (9) D. Weigmann, Reduction of disulfide bonds in keratin with 1,4-dithiothreitol. I. Kinetic investi- gation, J. Polymer Sci. 6, 2237 (1968). (10) L. J. Wolfram and D. L. Underwood, The equilibrium between the disulfide linkage in hair keratin and sulfite or mercaptan, Text. Resh. J., 36, 947 (1966). (11) M. S. Robinson and B. J. Rigby, Thiol differences along keratin fibers: Stress/strain and stress- relaxation behavior as a function of temperature and extension, Text. Resh. J., 55, 597 (1985). (12) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 2nd ed., (Springer-Verlag, New York, 1988), pp. 1-98. (13) C. Zviak, The Science of Hair Care, 2nd ed. (Marcel Dekker, New York, 1986), pp. 183-209. (14) F. J. Wortman and I. Souren, Extensional properties of human hair and permanent waving, J. Soc. Cosmet. Chem., 38, 125 (1987). (15) J. B. Speakman, Proc. Royal Soc. (London), 103B, 377 (1928). (16) J. B. Speakman and S. Y. Shah, The plasticity of animal fibres, J. Soc. Dyers Colourists, 57, 108 (1941). (17) M. Feughelman and B. J. Rigby, A two energy state model for the stress relaxation and creep of wool fibres in water, Proc. Int. Wool Textile Res. Conf. Australia, D62 (1955). (18) G. C. Wood, J. Textile Inst., 45, T462 (1954). (19) A. Sch6berl and H. Gr•ifje, Ober Disulfid-Austauschreaktionen bei hieder- und hochmolekularen Verbindungen, Fate SeiJ•n Anstrich., 60, 1057 (1958). (20) P. Bor• and J. C. Arnaud, Actualites Dermopharm., 6, 75 (1974).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)