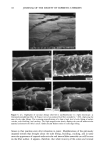
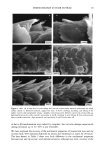
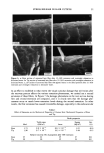
DISULFIDE BOND REDUCTION IN HAIR 55 DISCUSSION EFFECT OF PH VARIATION ON THE REACTION RATE CONSTANT OF HAIR REDUCED BY ATG It has been established that the redox potentials of thiol compounds increase with pH and that increasing the pH of the solution also affects the reaction rate constant, k. This trend has been shown by Wickett for reduction of human hair by sodium thioglycolate (1) and by Weigmann for the reduction of wool fibers by 1,4-dithiothreitol (9). Sim- ilarly, in this study, the reduction of human hair by ammonium thioglycolate was found to be dependent on pH (Table I). A 1 M ATG solution reduced hair more rapidly at pH 9.5 (which is nearest the pKs• of 10.4) than the same solution at either pH 9.0 or 8.0. Sodium thioglycolate has been observed to exhibit pseudo first-order kinetic behavior at pH • 10 (1). Ammonium thioglycolate, which differs from sodium thioglycolate only by the type of cation, also exhibited pseudo first-order kinetic behavior at pH • 10. EFFECT OF DITHIODIGLYCOLIC ACID ON REACTION RATE CONSTANT OF HAIR REDUCED BY ATG The effect of the addition of DTDG on the reaction rate constant of hair reduced by ATG was studied in order to determine if the reduction of keratin was affected. More spe- cifically, this condition was investigated to determine if DTDG was a competitive or blocking agent for the disulfide interchange mechanism. Speakman et al. (15,16) have demonstrated that stress relaxation of keratin fibers occurs in two distinguishable steps. The first step is the breakage of hydrogen bonds, van der Waals interactions, and salt linkages (17,18). The second step is the rate-determining breaking of disulfide bonds. Weigmann et al. (5,7) have shown that in the presence of both reducing agents and increased temperature disulfide bonds are transformed into energetically favored positions (i.e., stress-free positions) through a disulfide inter- change reaction with sulfhydryl groups. Furthermore, Weigmann et al. (5,7) have demonstrated by use of an SH-blocking reagent that the disulfide interchange mecha- nism dominates the second step of stress relaxation. Therefore, in the presence of a blocking reagent, the interchange mechanism cannot proceed, and structural rearrange- ment leading to stress relaxation does not occur. This would be observed as a decrease in the rate of stress relaxation of the fiber. The results from this present study indicate the effects of addition of DTDG on the kinetics of stress relaxation of hair by 1 M ATG at pH 9.0 are not significant. From the observed reaction-rate constants for hair fibers reduced under immersion conditions having 1.5% strain applied, the effect produced by the addition of 0. 125 M DTDG on the reaction rate constant was not statistically significant. The same was true for the addition of 0.250 M DTDG to a ! M ATG solution at pH 9.0. At low extension rates, stress is almost exclusively supported by the disulfide bonds in the fiber, which are displaced by an interchange mechanism with existing thiol groups into stress-free positions (5). This interchange mechanism results in rapid stress relax- ation of the fiber. If DTDG behaved as a blocking or competitive reagent, a decrease in the reaction-rate constant for stress relaxation would have been observed. Therefore, we propose that DTDG is not a competitive reagent, as the addition of DTDG to solutions of ATG does not affect the kinetics of stress relaxation when the fiber is held under !. 5 %
56 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS strain, and as all three treatments exhibited pseudo first-order kinetics and similar reaction rate constants were observed. MECHANISM FOR REDUCTION OF HAIR BY AMMONIUM THIOGLYCOLATE WITH DITHIODIGLYCOLIC ACID The mechanism of disulfide reduction by mercaptans (thioIs) has been shown to proceed through two displacement reactions (Eqs. la and lb). In 1958, SchiSberl and Griifje (19) proved that the first displacement reaction (Eq. la) leads to the formation of the mixed disulfide ofkeratin and mercaptan. Later, in 1974, Bor6 and Arnaud (20) confirmed that nucleophilic substitution occurs in the second displacement reaction (Eq. lb) and in- dicated that another pathway may exist (Eq. 6): Ker-S-S-R + KerS- • R-S-S- + Ker-S-Ker --• R-S- + S + Ker-S-Ker mixed disulfide lanthionine lanthionine (6) The mechanism of reduction of hair by a solution of ammonium thioglycolate with dithiodiglycolic acid is thought to be more complex as the number of intermediate pathways is increased. Upon examination of such a system, the potential competitive reduction pathways for this system may be proposed (Scheme 1). The first nucleophilic substitution by the mercaptide ion, RS, may proceed through either of two pathways, given as (A) or (B). The pathway designated as (A) resembles that of Equation la, while (B) is simply a monomer-dimer interchange. Both pathways (A) and (B) may participate in the second nucleophilic substitution by a second mercaptan ion as given by (A•), (A2) , (B•), and (B2). The pathway designated (A•) resembles Equation lb, while pathway (A2) is a monomer-dimer interchange. Pathway (B •) is the same as (A), whereas pathway (B2) is nothing more than a monomer-dimer interchange. Theoretically, the probability of the monomer-dimer interchange pathway predomi- nating should increase as the concentration of DTDG in the solution is increased. However, this is not the case, as the monomer-dimer interchange is not the rate- determining step of the reaction. According to the pseudo first-order kinetic model, the rate-determining step is the slow reduction of the disulfide bonds in keratin by the reducing agent. Therefore, the monomer-dimer interchange pathway may be neglected in this system, and the system simplifies to that of the reduction of keratin by a mercaptan as given by Equations la and lb. EFFECT OF ADDITION OF DITHIODIGLYCOLIC ACID ON FIBER STRENGTH In order to determine if the addition of DTDG to an ATG solution limits the extent of fiber reduction, the fiber strength was assessed after various times (5, 10, or 15 minutes) using the 20% index. The 20% index ratio is a measure of the force at 20% extension after treatment versus the force at 20% extension before treatment. Values of the 20% index near 1 indicate that the fiber is essentially unchanged, while values near zero indicate a greater degree of fiber weakening. The results (Table III) indicate that similar degrees of fiber weakening occurred for all three treatments at equivalent times. This indicates that the extent of stress-supporting disulfide bond reduction was not limited as determined by SFTK results when the fiber was held under 1.5% strain.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)