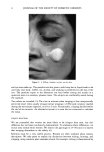
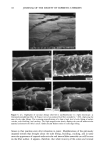
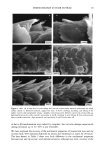
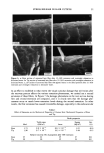
DISULFIDE BOND REDUCTION IN HAIR RESULTS THE EFFECT OF pH VARIATION ON REACTION RATE CONSTANT OF HAIR REDUCED BY ATG The relationship between tensile strength and the number of disulfide bonds in the hair fiber can be used to study the effects of pH variation on the reaction rate constants and kinetics (1-11). Since it has been established that the Hookean region of the stress/strain diagram is sensitive to the degree of hydrogen bonding in the fiber, a pre-stretch in water or a buffer solution to a constant level of force would effectively break the hydrogen bonding network in the hair. Once this network has been removed, the assumption that the tensile strength of the fiber is proportional to the number of disulfide bonds remaining may be made (1-4). By monitoring the stress relaxation of the fiber in response to cleavage of disulfide bonds, the reaction rate constant and kinetic behavior may be determined. With this relationship in mind, the effects of reduction by 1 M ATG solutions at pH 8.0, 9.0, and 9.5 at 23øC were studied by collecting stress-relaxation data. Graphs of the ln[(Ft-Ff)/(Fo-Ff)] versus time indicated that reduction by a I M ATG solution at pH 8.0, 9.0, and 9.5 at 23øC exhibits pseudo first-order kinetics. The reaction rate constant, k, for each condition was determined from the slopes of plots of ln[(Ft-Ff)/(Fo-Ff)] versus time, and the mean value was calculated. The results of the evaluation of the effects of reduction by 1 M ATG solutions at various pH levels on the reaction rate constants are indicated in Table I. From analysis of the results, the trend of increasing rate of reaction as the pH of the solution increases is apparent. This pH trend for ATG was similar to the results reported by Wickerr (1-3) for sodium thio- glycolate at pHs below 10. THE EFFECT OF DITHIODIGLYCOLIC ACID ON REACTION RATE CONSTANT OF HAIR REDUCED BY ATG The effect of addition of increasing amounts of dithiodiglycolic acid to 1 M ATG solutions at pH 9.0 at 23øC on the rate of reaction was investigated by monitoring stress-relaxation behavior. Table II contains the results of stress-relaxation measurements of three different treatments performed on 30-mm hair fiber segments. From analysis of the data using the paired t-test, the effect of DTDG on the reaction rate constant was not significant when the fiber was reduced under 1.5% constant strain. Graphs of the ln[(F•-Ff)/(Fo-Ff)] versus time indicated that pseudo first-order kinetic behavior of 1 M ATG, pH 9.0, 23øC, was unchanged by addition of dithiodiglycolic acid. Table I Reaction Rate Constants for Hair Fibers Reduced With 1 M Ammonium Thioglycolate at pH 8.0, 9.0, or 9.5 at 23øC k* 103 (s- •) Treatment (pH) (Mean, SD) 8.0 1.52, 0.35 9.0 4.57, 1.59 9.5 6.29, 0.46
54 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table II Reaction Rate Constants for Hair Fiber Segments Reduced With Either 1 M ATG, 1 M ATG q- 0. 125 M DTDG, or 1 M ATG + 0.250 M DTDG at pH 9.0 at 23øC Treatment k* 103 (s- t) (Mean, SD) 1 M ATG 1M ATG q- 0.125 MDTDG 1 M ATG q- 0.250 M DTDG 4.41, 0.31 4.48, 0.24 3.42, 1.08 ATG, ammonium thioglycolate. DTDG, dithiodiglycolic acid. THE EFFECT OF ADDITION OF DITHIODIGLYCOLIC ACID ON FIBER STRENGTH To investigate the effect of DTDG on the degree of fiber weakening, fibers from the same source were again divided into three 30-mm treatment sites that were rotated among treatment groups. The fibers were reduced for 5, 10, or 15 minutes in 10 ml of solution and rinsed with distilled water. The mean 20% index was then determined for each treatment. The results of these measurements (Table III) indicated that similar degrees of fiber weakening resulted when the fibers were reduced for 5, 10, or 15 minutes under 1.5% constant strain without neutralization. The effect of DTDG on fiber strength after neutralization was also investigated. Fibers were reduced for 5, 10, or 15 minutes, rinsed in distilled water, and neutralized for 5 minutes. The results of the mean 20% index determination for each treatment group (Table III) indicated that addition of DTDG affects the strength of the fiber after neutralization. For the fibers that were reduced for 5 or 10 minutes and neutralized, the results indicated that fiber strength increased as the concentration of DTDG added to the reducing solution was increased. However, fibers that were reduced for 15 minutes and neutralized exhibited 20% index values that were not significantly different. Table III Comparison of 20% Index for Hair Fibers Reduced With Either 1 M ATG, 1 M ATG q- 0.125 M DTDG, or 1 M ATG +0.250 M DTDG at pH 9.0 and 23øC for a Specific Time Length 20% Index 20% Index Treatment Reduction time (min) without neutralizer with neutralizer 5 0.70 + 0.08 0.68 -+ 0.05 10 0.40 - 0.04 0.61 --- 0.05 15 0.36 -+ 0.04 0.70 + 0.14 5 0.63 - 0.11 0.80 + 0.09 10 0.42 -+ 0.04 0.71 - 0.04 15 0.40 --+ 0.02 0.73 -+ 0.09 5 0.64 --+ 0.08 0.79 --+ 0.17 10 0.43 --+ 0.05 0.75 --+ 0.08 15 0.41 --+ 0.03 0.72 --+ 0.15 All values reported are the mean of six measurements: Treatment 1:1 M ammonium thioglycolate. Treatment 2:1 M ammonium thioglycolate + 0.125 M dithiodiglycolic acid. Treatment 3:1 M ammonium thioglycolate + 0.250 M dithiodiglycolic acid.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)