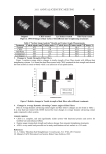
PANTHENYL TRIACETATE TRANSFORMATION 11 as for maintenance of the epidermal permeability barrier. Cholesterol synthesis in the epidermis is correlated with changes in mRNA levels for key enzymes, such as the HMG- CoA synthase family and HMG-CoA reductase. Mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMGCS2), an enzyme part of the HMG-CoA synthase family, catalyzes the fi rst step in isoprenoid/mevalonate synthesis and under some conditions controls the fl ux into the pathway (15,16). The capacity of both PTA and PAN to stimu- late HMGCS2 (+201% and +220% respectively, after 24 hours) is impressive. In the context of lipid synthesis and cholesterol it is also interesting to highlight the capacity of both PTA and PAN to stimulate cholesterol sulfotransferase (SULT2B1), thus infl uenc- ing cholesterol modifi cation. Also, in this case this enzyme has been linked to skin dif- ferentiation by infl uencing the amount of cholesterol sulfate and its binding to differentiation receptors such as PPAR and LXR (17-19). SULT2B1 has also been associ- ated with the sulfonation of cytotoxic oxysterols and therefore could have a role in skin detoxifi cation (20). The activation of SULT2B1 by both PTA and PAN suggests a new role for these vitamins as prodifferentiation and detoxifi cation agents. The increase in enzymes such a glucose phosphate isomerase (GPI) and glucose-6-phos- phate dehydrogenase (G6PD), representatives of glycolysis activity, was only observed when PTA was used and only after 24 hours. It is possible that activation of glycolysis to produce more piruvic acid is necessary to “feed” the energetic and metabolic pathways downstream (citric acid cycle and lipogenesis). It is also possible that PTA, providing a longer-term bioavailability of PAN, sustains a later activation of the glycolysis pathway only detectable at 24 hours and possibly lasting even longer, while the available PAN is consumed earlier in the skin metabolism. The data suggest PTA as a possible long-lasting reservoir for feeding PAN to the skin. We have, indeed, some previous evidence that treatment with PTA from persons with oily skin provided a long-lasting effect even when treatment was discontinued (8), sug- gesting a longer bioavailability of PTA-originated PAN. Finally, in order to correlate the metabolic activity of PTA and PAN with their capacity to heal and reduce TEWL after skin injury, we have conducted a clinical study comparing the two ingredients. PAN’s capacity as a skin-healing agent (2,21) has been mainly re- lated to its activity on fi broblasts (1) and on keratinocytes (5,6). In effect, we have con- fi rmed that enzymes involved in skin differentiation and the building of the skin lipid barrier, such as HMGCS2 and SULT2B1, are strongly stimulated by both PTA and PAN, although we don’t know whether there would also be an effect by PTA and PAN on fi bro- blasts in the healing process. The results of our clinical study (Figure 8) indentifi ed PTA as the only signifi cant treatment when compared to saline at 72 hours. It is intriguing to detect this effect at a late time point, i.e., after 72 hours of treatment from wound induc- tion. Also, in this case it can be postulated as a long-term effect from PTA. It is possible that a sustained action would be necessary to stimulate an added value of PTA when compared to a placebo or a PAN treatment in the context of wound healing. CONCLUSIONS We have demonstrated in vivo that D-panthenyl triacetate (PTA) can penetrate the human skin deeply and be transformed into D-panthenol. We have further demonstrated, in human skin explants, the activation by PTA of the enzymes involved into metabolic
JOURNAL OF COSMETIC SCIENCE 12 pathways such as gycolysis, the citric acid cycle, isoprenoids, and lipid synthesis. Finally, we have shown in a clinical study for wound healing and by transepidermal water loss (TEWL) that PTA can signifi cantly decrease TEWL when compared to a saline treat- ment, further showing its biological activity in vivo. We believe that D-panthenyl triacetate can be a valid active to incorporate in modern cosmetic formulations to target sensitive and atopic dry skin, to rebalance excessive skin sebum release, to reinforce the skin barrier, and to repair skin damage. ACKNOWLEDGMENTS The authors thank Mrs Parand Salmassinia for kindly reviewing and editing the manuscript. REFERENCES (1) F. Ebner, A. Heller, F. Rippke, and I. Tausch, Topical use of dexpanthenol in skin disorders, Am. J. Clin. Dermatol., 3, 427–433 (2002). (2) F. C. Combes and R. Zuckerman, Panthenol: Its topical use in cutaneous ulceration, J. Invest. Dermatol., 16, 379–381 (1951). (3) L. F. Eichenfi eld, J. F. Fowler, Jr, D. S. Rigel, and S. C. Taylor, Natural advances in eczema care, Cutis, 80, 2–16 (2007). (4) K. Biro, D. Thaçi, F. R. Ochsendorf, R. Kaufmann, and W. H. Boehncke, Effi cacy of dexpanthenol in skin protection against irritation: A double-blind, placebo-controlled study, Contact Dermatitis, 49, 80–84 (2003). (5) E. Proksch and H. P. Nissen, Dexpanthenol enhances skin barrier repair and reduces infl ammation after sodium lauryl sulphate-induced irritation, J. Dermatol. Treat., 13, 173–178 (2002). (6) W. Gehring and M. Gloor, Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study, Arzneimittelforschung, 50, 659–663 (2000). (7) G. Dell’Acqua, K. Schweikert, and G. Calloni, Oak, green tea and orange derivatives to disrupt JAK/ STAT, NF-κB irritation pathways, Cosmet. Toiletr., 126, 30–38 (2011). (8) W. McGregor, K. Schweikert, and G. Dell’Acqua, Sebum reduction in oily skin individuals by treat- ment with a combination of panthenyl triacetate and farnesyl acetate, precursors of the isoprenoid and sterol syntheses, SÖFW J., 132, 2–7 (2006). (9) B. Lacroix, E. Didier, and J. F. Grenier, Role of pantothenic and ascorbic acid in wound healing pro- cesses: In vitro study on fi broblasts, Int. J. Vitam. Nutr. Res., 58, 407–413 (1988). (10) V. S. Slyshenkov, K. Piwocka, E. Sikora, and L. Wojtczak, Pantothenic acid protects jurkat cells against ultraviolet light-induced apoptosis, Free Rad. Biol. Med., 30, 1303–1310 (2001). (11) V. S. Slyshenkov, D. Dymkowska, and L. Wojtczak, Pantothenic acid and pantothenol increase biosyn- thesis of glutathione by boosting cell energetic, FEBS Lett., 569, 169–172 (2004). (12) K. Schweikert, F. Gafner, and G. Dell’Acqua, A bioactive complex to protect proteins from UV-induced oxidation in human epidermis, Int. J. Cosmet. Sci., 32, 29–34 (2010). (13) T. Wiederholt, R. Heise, C. Skazik, Y. Marquardt, S. Joussen, K. Erdmann, H. Schröder, H. F. Merk, and J. M. Baron, Calcium pantothenate modulates gene expression in proliferating human dermal fi bro- blasts, Exp. Dermatol., 18, 969–978 (2009). (14) F. P. Schmook, J. G. Meingassner, and A. Billich, Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption, Int. J. Pharm., 215, 51–56 (2001). (15) R. Schmidt, E. J. Parish, V. Dionisius, C. Cathelineau, S. Michel, B. Shroot, A. Rolland, A. Brzokewicz, and U. Reichert, Modulation of cellular cholesterol and its effect on cornifi ed envelope formation in cultured human epidermal keratinocytes, J. Invest. Dermatol., 97, 771–775 (1991). (16) J. L Goldstein and M. S. Brown, Regulation of the mevalonate pathway, Nature, 343, 425–430 (1990).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)