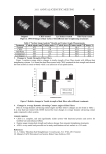
J. Cosmet. Sci., 63, 15–25 (January/February 2012) 15 Effects of oxidative treatments on human hair keratin fi lms T. FUJII, Y. ITO, T. WATANABE, and T. KAWASOE, Faculty of Textile Science and Technology, Shinshu University, 3-15-1 Tokida, Ueda-shi, Nagano, 386-8567 (T.F., Y.I.), and Shiseido Co., Ltd, Research Center, 2-2-1, Hayabuchi, Tsuzuki-ku, Yokohama-shi, Kanagawa, 224-8558 (T.W., T.K.), Japan. Accepted for publication July 20, 2011. Synopsis The effects of hydrogen peroxide and commercial bleach on hair and human hair keratin fi lms were examined by protein solubility, scanning electron microscopy (SEM), immunofl uorescence microscopy, immunoblot- ting, and Fourier-transform infrared spectroscopy. Protein solubility in solutions containing urea decreased when the keratin fi lms were treated with hydrogen peroxide or bleach. Oxidative treatments promoted the urea-dependent morphological change by turning fi lms from opaque to transparent in appearance. Immuno- fl uorescence microscopy and immunoblotting showed that the oxidation of amino acids and proteins occurred due to the oxidative treatments, and such occurrence was more evident in the bleach-treated fi lms than in the hydrogen peroxide-treated fi lms. Compared with hair samples, the formation of cysteic acid was more clearly observed in the keratin fi lms after the oxidative treatments. INTRODUCTION Human hair is generally classifi ed into three layers. From the outside inward, it is com- prised of cuticle, cortex, and medulla. The cortex, which occupies 80% or more of the total mass, is constructed of a hierarchical fi brous structure mainly consisting of keratin fi laments and keratin-associated proteins (KAPs). Since the cysteine contents in keratin and KAPs are considerably higher than in other proteins (1,2), the cysteine residues of these proteins can easily crosslink and form intermolecular and intramolecular covalent bonds. Thus, keratin and KAPs are considered to be closely related to the physical and mechanical properties of the hair. In order to create straight, curly, or colored hair styles, human hair usually goes through chemical treatments and heating. During these processes, the disulfi de crosslinks among the fi brous structures of keratin and KAPs are occasionally cleaved and recombined at different sites. Such oxidation and reduction of hair and its proteins is responsible for hair damage (2,3). Amino acid analysis showed that the cystine, methionine, and tyrosine contents of human hair decreased after bleach treatment, while the amount of cysteic acid increased (4,5). Nagai et al. (6) indicated a possibility that the isoelectric points of human hair proteins are changed when hair is treated by bleaching, dyeing, or perming. Re- cently, Plowman et al. (7) reported that oxidative treatment of wool had little effect on the
JOURNAL OF COSMETIC SCIENCE 16 isoelectric points of keratin type I and II components measured by two-dimensional gel electrophoresis, though the isoelectric points and spots of KAP components consisting of high glycine–tyrosine proteins and high-sulfur proteins were changed by the treatment. FT-IR and Raman analyses indicated that keratin fi bers in hair were disordered by oxida- tive and reductive treatments (8,9). These results suggest that these treatments on human hair will affect the higher order structure of hair through conformational changes in the fi brous proteins. We developed novel procedures for preparing human hair protein solutions and the pro- tein fi lms from them using the self-assembly of α-keratin (10–12). The conversion ratios from the solutions to the fi lms were 60–80%, and the fi lms mainly consisted of α-keratin types I and II and KAPs. Any signifi cant degradation was not observed during the prep- aration process and after storage for several months. SEM observations showed that the protein fi lms consisted of particles and fi lamentous structures (11,12). However, regular fi lamentous architectures like the hair cortex were not observed. Recently, we developed a sensitive method to detect UV-induced photodegradation in hair proteins using fl uores- cent microscopy (13). The sensitivity was signifi cantly higher when hair keratin fi lm prepared by the pre-cast method was used instead of hair samples. This indicates that hair keratin fi lms can serve as an alternative cortex fi lament to evaluate hair damage caused by protein degradation. The effect of oxidative treatments on hair keratin fi lms have not been reported. In the present study, we report the effects of hydrogen peroxide and commercial bleach on hair keratin fi lms. The morphological and biochemical properties of the keratin fi lm and its proteins were examined in detail. EXPERIMENTAL PREPARATION OF HUMAN HAIR KERATIN FILMS Human hair protein solution was prepared according to the “Shindai method,” which has been previously described by us (10). Briefl y, ethanol-treated hair samples that had not undergone chemical treatments such as bleaching or permanent waving were extracted using a solution containing 20 mM Tris-HCl (pH 8.5), 2.6 M thiourea, 5 M urea, and 150 mM dithiothreitol (DTT) at 50°C for 24 hr. This solution was added to hair at 60 mg/ml. After fi ltration and centrifugation, about 70% of the solution was recovered as extracted protein solution, which was then used for preparation of the pre-cast fi lm (11). The hair protein solution was mixed with acetic acid to a fi nal concentration of 20 mM and then poured into tissue culture dishes (diameter 40 mm) containing distilled water. A membrane-like aggregate was formed that spread out on the bottom of the dishes. After being washed with tap water for 36 hr and distilled water for 6 hr, the human hair keratin fi lms were recovered after drying at room temperature. OXIDATIVE TREATMENTS OF THE HAIR KERATIN FILMS AND PROTEIN SOLUBILIZATION The oxidative treatments of the hair keratin fi lms were done with solutions containing 0–10% hydrogen peroxide and 50 mM Tris-HCl (pH 8.5) at 25°C. The average weight
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)