OXIDATIVE TREATMENTS OF HAIR KERATIN FILMS 21 Figure 3. Changes in appearances of the hair keratin fi lms after various treatments and SEM observations. (A) The hair keratin fi lms were incubated with distilled water (a,d), 1% hydrogen peroxide (b,e), and bleach solution (c,f) at 25°C for 10 min. The fi lms (d–f) were further incubated with solution B at 50°C for 3 hr. (B) After washing and drying, the fi lms were used for morphological observations. Bars: 20 mm (A) 10 μm (B). peroxide-treated fi lm apparently remained unchanged. These fi lms were incubated with solution B at 50°C for 3 hr, washed with distilled water, and dried. Proteins in the un- treated fi lm partly dissolved and became translucent, making the letters “keratin” under- neath the fi lm visible. On the other hand, proteins in the fi lms treated with hydrogen peroxide or the bleaching agent hardly solubilized, resulting in their clearly unchanged appearances (Figure 3A, panels d, e, f). We reported that the surfaces of hair protein fi lms prepared by pre- and post-cast methods were composed of porous structures with fi ne fi laments and particles (11,12). SEM observa- tion showed that the surfaces of hair keratin fi lms prepared by the pre-cast method are also composed of porous structures containing small particles (Figure 3B, panels a, b, c). Such small particles on the surface of the translucent fi lms incubated with solution B disap- peared, while smooth porous structures remained (Figure 3B, panel d). Urea treatment ap- parently could not affect the fi ne structures of the fi lms, even after the oxidative treatments.
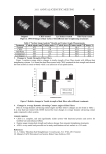
JOURNAL OF COSMETIC SCIENCE 22 We have not measured the amounts of cysteine sulphydryl and disulfi de bonds in the hair keratin fi lms used. While the disulfi de bonds of native hair proteins are broken during the protein extraction, most of the hair proteins are recovered as sulphydryl forms. When the hair proteins are put under the conditions of high protein concentrations, and when the denaturant and reductant are removed slowly from the solution, the disulfi de bond is al- lowed to reform, which leads to protein aggregates such as a fi lm. Thus, it is likely that cysteine remains in the fi lm to some degree and that the oxidation of it decreases the sensitivity of the fi lm to urea extraction. OXIDATION OF PROTEIN MEASURED BY FLUORESCENT MICROSCOPY AND IMMUNOBLOTTING When human hair and the hair keratin fi lms were exposed to UV irradiation, the forma- tion of oxidized protein was detected by a fl uorescent microscopy (13,15) using 5-FTSC, a specifi c reagent which binds to carbonyl groups of the hair proteins. We noted the pres- ence of carbonylated proteins in the fi lms after treatments of 1% hydrogen peroxide or the bleaching agent (Figure 4A). Compared to the fi lm treated with distilled water, a bright green color was observed in areas after the oxidative treatments. When the fl uores- cence intensity of the fi lms was calculated by image analysis, the highest intensity was found in the bleach-treated fi lm (Figure 4B). Next, we examined oxidized proteins by immunoblot analysis (Figure 5). As shown in Figure 5A, a signifi cant difference in the protein component, which consisted of keratin types I and II and KAPs from hair fi ber and the keratin fi lms, was not observed. The Figure 4. Fluorescence image and intensity of hair keratin fi lms treated with hydrogen peroxide or bleach- ing agent. (A) After treatment with distilled water (a), 1% hydrogen peroxide (b), or bleaching agent (c) at 25°C for 10 min, the hair keratin fi lms were reacted with 20 μM 5-FTSC and observed using fl uorescent microscopy. (B) The average of the fl uorescence intensity was calculated from 40 areas per one fi lm by image analysis.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)