OXIDATIVE TREATMENTS OF HAIR KERATIN FILMS 17 of the fi lms used was 18 mg/dish, and the solution was added at 2.5 ml/dish. After incu- bation for 0–10 min, the solutions were discarded and the fi lms were washed with tap water for 10 min and then with distilled water for 6 min. The fi lms were dried at room temperature and used. Additional hair protein fi lms were bleached using a commercial bleaching agent (UNO hard bleach, Shiseido, Japan), following the manufacturer’s in- structions, at 25°C for 10 min. The bleaching agent contained 2% ammonium hydrox- ide, 1.5% monoethanolamine (pH 10.1), 3% H2O2, and potassium persulfate. The fi lms were then similarly rinsed and dried. Then, the fi lms were collected, ground to powders, and used in the following experiments. Solubilization of proteins from the untreated, oxidized, or bleached keratin fi lms was determined using solution A (50 mM Tris-HCl, pH 8.5), solution B (50 mM Tris-HCl, pH 8.5, containing 8 M urea), solution C (50 mM Tris-HCl, pH 8.5, containing 5 mM DTT), and solution D (50 mM Tris-HCl, pH 8.5, containing 8 M urea and 5 mM DTT). The powdered fi lms were mixed with solutions A, B, C, or D at 5 mg/ml and incubated at 50°C for 3 hr. After centrifugation at 12,000g for 5 min, the supernatants were recov- ered in test tubes and used for measurement of the protein concentration and for electro- phoresis. The protein concentrations were determined according to Bradford, using bovine serum albumin as the standard (14). SEM OBSERVATION The hair keratin fi lms before and after the treatments with hydrogen peroxide, the bleach- ing agent, or solution B were sputtered with platinum, and the fi ne structures of the fi lms was observed by scanning electron microscopy (Neoscope JCM-5000, JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 20 kV. FLUORESENCE MICROSCOPY The hair keratin fi lms were treated with distilled water, 1% hydrogen peroxide, or the bleaching agent at 25°C for 10 min. Then the fi lms were incubated with 3 ml of the staining solution, which consisted of 20 μM fl uorescein-5-thiosemicarbazide (5-FTSC) in 100 mM 2-morpholinoethane sulfonic acid-NaOH (pH 5.5) at 25°C for 2 hr (15). To remove non-reacting 5-FTSC, the fi lms were rinsed with 0.1% SDS, 300 mM NaCl, and 30 mM sodium citrate buffer (pH 7.0) for 30 min at 50°C, then in 0.1% SDS, 30 mM NaCl, and 3 mM sodium citrate buffer (pH 7.0) for 30 min at 25°C, and fi nally in distilled water for 10 min at 25°C. After rinsing, the fi lms were dried at room temperature. All procedures were carried out in a dark room. Afterward, the fi lms were observed and photographed using fl uorescence microscopy (VB-G25, Keyence, Japan). GEL ELECTROPHORESIS Sodium dodecyl sulfate-polyacrylamaide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (16) using a 5-20% gradient polyacrylamide gel. After the electrophoresis, proteins in the gel were stained with 0.1% Coomassie brilliant
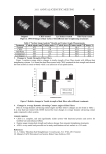
JOURNAL OF COSMETIC SCIENCE 18 blue R-250, 10% acetic acid, and 40% ethanol for 1–2 hr. Destaining was carried out in 10% acetic acid and 40% ethanol. IMMUNOBLOTTING The proteins were extracted by incubating the untreated, 1% hydrogen peroxide-, or bleach-treated keratin fi lms and hair samples with 50 mM Tris-HCl (pH 8.5) containing 8 M urea and 5% 2-mercaptoethanol at 50°C for 20 hr. Proteins separated by 5-20% SDS-PAGE were electrophoretically transferred to nitrocellulose fi lters and washed with PBS (phosphate-buffered saline). The fi lters were reacted with dinitrophenyl (DNP) hy- drazine at 25°C for 15 min (OxyBlotTM Protein Oxidation Detection Kit, Chemicon, U.S.A. and Canada). After incubation of the fi lters at 4°C overnight with reagent N-102 (NOF Corporation, Tokyo Japan) to block non-specifi c binding sites, they were incubated with 1/150 anti-DNP antibody for 1 hr at 25°C. After a rinse with PBS containing 0.1% Tween 20, the fi lters were reacted with 1/300 peroxidase-conjugated anti-rabbit IgG for 1 hr at 25°C. Then, the fi lters were washed with PBS containing 0.1% Tween 20, and the antibody binding was visualized using the Super Signal West Trial Kit (Thermo, Japan). FOURIER-TRANSFORM INFRARED (FT-IR) MEASUREMENT FT-IR spectra were obtained from the fi lms and hair samples before and after treatment with 1% hydrogen peroxide or the bleaching agent. The fi lms and hair samples were ground to fi ne particles with an agate mortar and pestle. The measurements were per- formed by attenuated total refl ectance (ATR) with an IR Prestige-21 (Shimadzu, Japan), collecting 50 scans at a resolution of 4 cm−1. RESULTS AND DISCUSSION PROTEIN ELUTION FROM THE HAIR KERATIN FILMS TREATED WITH OR WITHOUT HYDROGEN PEROXIDE AND BLEACH When the human hair keratin fi lms prepared by the pre-cast method were incubated with 1-5% hydrogen peroxide solution or the bleaching agent at 25°C for 10 min, the fi lm weights after these treatments were little changed compared to those of the untreated fi lms. These results suggest that the protein fi lms can withstand the oxidative treatment. Four kinds of solutions (pH 8.5) containing urea and DTT were used to examine the protein solubility from the keratin fi lms treated with 1% hydrogen peroxide solution or bleach at 25°C for 10 min. Little protein from the untreated, hydrogen peroxide-, or bleach-treated fi lms was dissolved by solution A (Figure 1). In solution B, containing 8 M urea, and solution C, containing 5 mM DTT, protein solubilization (5–10%) was similar for the untreated fi lms. Interestingly, the amount of protein solubilization from the hydrogen peroxide-treated fi lms was decreased to almost zero in solution B, while the amount was little changed in solution C. The amount of protein solubilization from bleach-treated fi lms in solution C was higher the expected, and this is considered to be the result of a protective hardening effect by the fi lms against oxidative treatment. When
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)