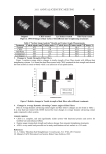
OXIDATIVE TREATMENTS OF HAIR KERATIN FILMS 23 Figure 5. Analysis of solubilized oxidative proteins from hair samples and keratin fi lms by immunoblotting. (A) CBB staining. (B) Immunostaining using anti-DNP antibody. (h, hair a, untreated fi lm b, H2O2- treated fi lm c, bleach-treated fi lm.) Figure 6. FT-IR spectra of keratin fi lms and hair samples with or without oxidative treatments After treat- ment with distilled water (—), 1% hydrogen peroxide (----), or the bleaching agent (—), the hair keratin fi lms (A) and hair samples (B) were recovered. Absorbance was normalized as 1 at the peak of 1076 cm-1 to compare the relative amounts of -SO3H observed at 1041 cm-1, which appeared prominently when using the keratin fi lms as an oxidized form of cysteine. protein samples extracted from hair fi ber and the keratin fi lms were reacted with DNP hydrazine, a specifi c reagent that binds to aldehydes from basic amino acids and threo- nine. After oxidative treatments of the fi lms, the reactivities of KAPs and high-molecular- weight components (HMPs) with a molecular mass more than 100 kDa were higher relative to the keratin bands (Figure 5B). The reactivity of high-molecular-weight com- ponents was particularly enhanced when the fi lms were treated with the bleach.
JOURNAL OF COSMETIC SCIENCE 24 FT-IR ANALYSIS Bleaching human hair causes a change in amino acid composition in the protein that is to say, as cysteine decreases, cysteic acid increases (5,17). The relative amounts of possible oxidation products of the S–S bond after oxidative treatments can be also determined by FT-IR measurement. In fact, the formation of cysteic acid was confi rmed in bleached hu- man hair (18,19). We examined by FT-IR measurement the formation of cysteic acid in hair samples and the keratin fi lms after oxidative treatments (Figure 6A,B). The absor- bance at the -SO3H (1041 cm−1) was compared, and after normalization of the absorbance at 1076 cm−1, in the untreated hair and fi lms, the peak was hardly detected. Upon com- parison of the hydrogen peroxide-treated hair and fi lms, the peak at 1041 cm−1 was scarcely detected in the hair, while it was clearly evident in the fi lms. The peaks were detected in the bleach-treated fi lms and hair however, the absorbance was fi ve to ten times higher in the bleached fi lms compared with that of bleached hair. These results suggest that an oxi- dized form of cysteine residues is easily identifi ed when hair keratin fi lms are used. CONCLUSION Human hair keratin fi lm does not have features such as trace metals and diffusion-modulating morphology that are present in hair fi bers. However, its use as an alternative cortex fi la- ment can be promising for the evaluation of hair damage by oxidative treatments contain- ing bleach. REFERENCES (1) G. L. Vuong, S. M. Weiss, and W. Kammer, Electrophoresis, 21, 2594–2605 (2000). (2) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 4th ed. (Springer-Verlag, New York, 2002). (3) M. L. Tate, Y. K. Kamath, S. B. Ruetsch, and H. -D. Weigmann, Quantifi cation and prevention of hair damage, J. Soc. Cosmet. Chem., 44, 347–371 (1993). (4) C. Robbins and C. Kelly, Amino acid analysis of cosmetically altered hair, J. Soc. Cosmet. Chem., 20, 555–564 (1969). (5) L. J. Wolfram, K. Hall, and I. Hui, The mechanism of hair bleaching, J. Soc. Cosmet. Chem., 21, 875–900 (1970). (6) A. Nagai, H. Komoriya, Y. Bunai, S. Yamada, X. Jiang, and I. Ohya, Effect of hair dyes and bleach on the hair protein patterns as revealed by isoelectric focusing, Electrophoresis, 12, 451–453 (1991). (7) J. E. Plowman, L. M. Flanagan, L. N. Paton, A. C. Fitzgerald, N. I. Joyce, and W. G. Bryson, The effect of oxidation of alkylation on the separation of wool keratin proteins by two-dimensional electrophoresis, Proteomics, 3, 942–950 (2003). (8) A. Kuzuhara, Analysis of structural changes in bleached keratin fi bers (black and white human hair) using Raman spectroscopy, Biopolymers, 81, 506–514 (2006). (9) C. Yamauchi, W. Okazaki, K. Inoue, and A. Sakaino, Enzymatic method for assessing hair damage with reduction and subsequent oxidation, Sen’i Gakkaishi, 63, 33–38 (2007). (10) A. Nakamura, M. Arimoto, K. Takeuchi, and T. Fujii, A rapid extraction procedure of human hair proteins and identifi cation of phosphorylated species, Biol. Pharm. Bull., 25, 569–572 (2002). (11) T. Fujii, D. Ogiwara, and M. Arimoto, Convenient procedures for human hair protein fi lms and proper- ties of alkaline phosphatase incorporated in the fi lm, Biol. Pharm. Bull., 27, 89–93 (2004). (12) T. Fujii and Y. Ide, Preparation of translucent and fl exible human hair protein fi lms and their properties, Biol. Pharm. Bull., 27, 1433–1436 (2004). (13) T. Kawasoe, T. Watanabe, and T. Fujii, Visualization of modifi ed human hair by artifi cial sunlight with carbonylated proteins as an indicator of hair damage, J. Jpn. Cosmet. Sci. Soc., 34(4), 287–291 (2010).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)