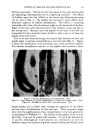
150 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ance values can already be detected. As long as no clinical criteria have been formulated and tested in the field, it must remain an open question how much protection has to be built into the formulation of an oral product in order to maintain the integrity of oral ]eukocytes. The leukocytes are classified in four phases of cytomorphosis (2), scribing functional and morphological changes simultaneously. The first phase of cytomorphosis pertains to the formative phase of leukocytes, which occurs in the case of polymorphonuclear ]eukocytes (neutrophils) in the bone marrow and will, therefore, not be found in the mucus of the oral cavity. The second phase of cytomorphosis shows signs of differentiation in morphology as well as in function. The cell cytoplasm and nucleus, rather large and nondescript during the first phase of cytomorphosis, start condensing into smaller and final mature size of between 12 and 15 u. The cell cytoplasm contains, in addition to a multi-lobed nucleus and a few mitochondria, a multitude of very small particles, the so-called specific granules of the leukocytes. These are the carriers of many hydrolytic enzymes, and 80 to 100 can be counted per cell. The cell shows active locomotion through the formation of pseudopods and is capable of en- gulfment of foreign matter. The third phase is reached when the cell grows older and loses its capacity for phagocytosis. Instead of polarized locomotion through active pseud- opod formation, only many rounded vesicles are formed by the cell mem- brane. The number of specific granules diminishes, the cell becomes senile and enters the fourth and last phase. All membrane and granular motion comes to a standstill, and the cell disintegrates, either by swelling and final disruption or by fragmentation, depending on the properties of the environment. This environment is the mucus secreted by the major and minor mucous glands of the oral cavity. The harvesting of oral leukocytes is, therefore, aimed at the collection of the mucus covering the mucous membranes of the oral cavity. The mucus which harbors millions of leukocytes can be exposed to the action of an oral product. All oral products investigated thus far evoke a leu- kocytic response, and all cause complete destruction of leukocytes at higher concentrations. The first method to be described is for a detailed microscopic study of leukocytic behavior. It enables the observer to watch especially the second phase leukocytes. Changes in the flow of specific granules with respect to pseudopod formation are indicative of changes in membrane permeability. A second phase leukocyte is, so to speak, prematurely rushed into the third phase. Some oral products may at very low con- centrations carry a second phase leukocyte into the fourth phase in a matter of seconds, resulting in complete cell disruption.
LEUKOCYTE DAMAGE BY ORAL PRODUCTS 151 The second method to be described is more suitable for the L.D.50 determination of an oral product. EXPERtMENTAL METHOD I. For Morphological Evaluation The composition and preparation of the harvesting solution has been described elsewhere (1). It is a modified isotonic balanced saline solution. If the oral product is a mouthwash, a certain volume is added to the harvesting solution to obtain the required percentage for the test. If the oral product is a toothpaste, the test concentration is obtained by adding the required volume of paste to the harvesting solution. Vigorous shaking is followed by centrifugation. The clear supernatant fluid is used as the harvesting solution. Five ml. of this solution is kept intra-orally for 30 seconds, expectorated, centrifuged at 1200 X g for 10 minutes, and the mucus sediment is examined with a phase contrast microscope. H. For counting and L.D.5o determination A different approach is used because the leukocyte count is of prime im- portance. A hypertonic solution is prepared by concentrating the harvest- ing solution 1.5 X. Used as an oral rinse, it shrinks the leukocytes, causing them to retract their pseudopods or vesicles and spherulizing them completely. The main purpose, however, is that mucus can be removed by vigorous shaking without causing plasmolysis. Even fourth phase leukocytes, not viable but still intact, are prevented from rupturing and remain available for the count. Five ml. normal isotonic harvesting solution is used for a 30-second rinse. The expectorated sample is divided into two equal parts, A and B. To part A fresh harvesting solution is added, up to 8 ml., to serve as a control. To part B fresh harvesting solution is added, up to 4 ml. followed by 4 ml. oral product-isotonic harvesting solution-extract of desired concentration. Samples A and B are then inverted three times to mix the mucus with the solutions without destroying the stretch fibrilla- tion and elastic recoil properties of the mucus. Samples A and B are centrifuged for 10 minutes. The supernatant fluids are then completely decanted. For the counting procedure, the 11/2 X concentrated solution is added to each of the mucus sediments, up to 6 mi. The hypertonicity introduced at this time insures the cell membrane integrity. Vigorous shaking for 15 seconds destroys the mucus properties of stretch fibrillation and elastic recoil. The samples lose their viscosity. The number of intact leukocytes in the A and B samples is determined with a standard A. O. Neubauer hemacytometer and a phase contrast microscope. The concentration at which the B count is 50% of the A count determines the L.D.50 of the product.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)