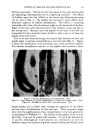
154 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS "miracle" ingredients built into the formulation of modern oral products is aimed at the achievement of cleaner and healthier conditions for the oral cavity. Their effect can now be determined on the leukocyte population. Leukocyte plasmolysis was to be expected from products containing surface tension diminishing substances. Fixation and fragmentation was predictable for those products containing protein coagulants. It was not known what to expect from products containing halogen and heavy metal-ion combinations. Of greatest interest is the possibility to devise a procedure whereby the specific effects of these additives can be evaluated as to their mucus penetrating powers and final effect on leukocytes actively engaged in their natural function ofphagocytosis. /lcknowledgment. The authors are grateful to Dirk L. Hajema for the com- petent technical assistance and skillful time lapse phase contrast cinerni- crographic recordings, used f'or this study. This investigation was supported by U.S. Public Health Service Grant #GM-06331. (Received October 1, 1963) REFERENCES (l) J.M. Klinkhamer, y. •tm Soc. Periodontists, 1, 109 (1963). (2) C. S. Minor, Laboratory Textbook of Embryology, P. Blakiston's Son & Co., Philadelphia, 1903.
J. soc. cos. CHEM. 15, 155-159 (1964) PRETESTING OF COSMETICS FOR CONSUM ER PROTECTION By DAVID J. MILLER, B.S.* Presented September 24-25, Z963, Seminar, Boston, Mass. ABSTRACT Recently proposed legislation contains a provision which requires cosmetics to be tested for safety before marketing. The require- ments for such safety testing of cosmetics and the effect of the Color Additive Amendments of the law on cosmetics in interstate commerce are discussed. Finally, it is emphasized that the Color Additives Regulations limit the coal tar hair dye exemption of the Food, Drug and Cosmetic Act to those hair colorings having sensitivity reaction only. This discussion will be concerned with over-all problems of pre- testing of cosmetics as they relate to safety considerations. Some members of the industry still recall the controversies and discussions that took place during the developmental period that led to the Food, Drug and Cosmetic Act of 1938 and may remember the vivid illustrations of dan- gerous cosmetics that were used during that period in supporting new cos- metic legislation. That Act contained provisions for pretesting of safety of drugs but did not contain a similar requirement for foods or cosmetics. Under that law, it was necessary for the Food and Drug Administration to demonstrate and to be prepared to prove in court that a cosmetic was dangerous before it could be removed from interstate commerce. All of us are aware, of course, of the Food Additives Amendment of 1958 which simulated the new drug requirement of 20 years earlier in establishing the requirements for pretesting of chemical substances used in foods prior to their being marketed. It is also likely that all of us are aware of the more recent legislation enacted in 1960 which requires the pretesting of the class of cosmetics which may be defined as color additives. Final regulations relating to color additives were published on June 22, 1963, and set forth the conditions that must be met in the marketing of cosmetics of this class. It may be recalled that in the regulations the term "color additive" is defined as "Any material.. that is a dye, pigment or other substance made by a process of synthesis or similar art}rice, or extracted, * Food and Drug Administration, Dept. of Health, Education, and Welfare, Washington 25, D.C. 155
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)