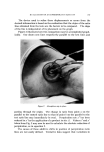
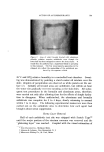
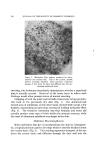
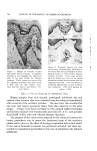
8OO (s) (9) (lO) (11) (12) (13) (14) (15) JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Papa, C. M., and Kligmau, A.M., Sweat pore patterns, J. Invest. Dermatol., 46, 193 (1966). Dobson, R. C., and Lobitz, W. C., Jr., Some histochemical observations on the human eccrine sweat glands. II. Pathogenesis of miliaria, Arch. Dermato[., 75, 653 (1957). Formisano, V., and Lobitz, W. C., Jr., The "Schiff-positive non glycogen material" in the human cccrine glands, Arch. Dermato[., 75,202 (1957). Randall, W. C., and Peiss, C. N., The relationship between skin hydration and the sup- pression of sweating, Y. Invest. DernmtoL, 28,435 (1957). Sarkany, I., Shuster, S., and Stammers, M. C., Occlusion of the sweat pore by hydra- tion, Brit. J. Dermatol., 77, 101 (1965). Sulzberger, M. B., Herrmann, F., and Zak, F. G., Studies of sweating. I. Preliminary report with particular emphasis on a sweat retention syndrome, J. Invest. Dermatol., 9, 221 (1947). Cormia, F. E., and Kuykendall, V., Studies in sweat retention in various dermatoses, Arch. Dermato[., 69,543 (1954). Papa, C. M., Personal observations.
J. Soc. Cosmetic Chemists, 17• 801-830 (196(i) Molecular Complex Formation in Aerosol Emulsions and Foams PAUL A. SANDERS, Ph.D.* Presented May 10, l•66, New York City Synopsis--Previous studies in nonaerosol systems have shown that certain detergents, such as sodium lauryl sulfate, form molecular complexes with long chain alcohols or acids at air/ water or oil/water interfaces. In the present investigation it has been shown that molecular complexes are also formed in aerosol emulsion systems. It was found that, in many cases, the addition of a long-chain alcohol to an aerosol emulsion system prepared with the tri- ethanolamine salt of a fatty acid or sodium lauryl sulfate as the surfactant produced a marked increase in emulsion and foam stability and a decrease in foam drainage. In some cases foam viscosity was increased. These effects of the long-chain alcohols occur in nonaerosol and aerosol systems and are indicative of molecular complex formation. INTRODUCTION It has been known for a long time that combinations of certain surfactants produce unexpected effects in emulsion systems. The reason for this remained obscure for many years, and in many cases the causes are still unknown. However, the effect of combinations of surfactants and long-chain polar compounds, such as the fatty alcohols, has been shown to be due to the formation of molecular complexes between the surfactants and the alcohols. The study of these molecular complexes has clarified much of the phenomena observed with these combinations. The investigation of molecular complexes was initiated in 1937 by Schulman and Rideal (1) with the discovery that sodium cetyl sulfate and cholesterol formed stable complexes at air/water interfaces. These complexes formed because of the attraction between the polar groups of * "Freon" Products Laboratory, E. I. du Pont de Nemours and Co., Wilmington, Del~ 801
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)