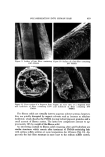
RHEOLOGICAL STUDIES AND PRODUCT FORMULATION 447 Theoretical (vol. x density) (vol. x density) (acceleration) minimum = (of particle) - (disp. medium) x (of gravity) yield value cross sectional area of particle While this is a simplistic approach, and does not consider many forces known to be at work in a suspension, it did afford these investigators a fairly reliable means of producing stable, permanent suspensions. The Carbopol resins emerged as the best suspending agents in this study, because they pro- duced a high yield value when used in low concentrations. Laboratory evaluation of a Carbopol©* 934 solution has shown that three types of yield value exist. This is illustrated in Fig. 6. Value B is the conven- tional or Bingham Value which is an extenson of the straight line portion of the curve. It is the most commonly cited value. Value A, which can be cal- culated, is the point at which it is presumed that laminar flow starts. Value A is sometimes referred to as the "Experimental Yield Value." This value can be obtained from measurements using a rotational viscometer. It is the same type of value reported earlier by Meyer and Cohen (13). Value C, which is perpendicular to the tangent is called calculated yield value and is very rarely used. *B. F. Goodrich Chemical Co., Cleveland, Ohio. R A T 0 F S H A R (s•.c 'x) A = EXPERIMENTAL YIELD VALUE B BINGHAM YIELD VALUE C CALCULATED YIELD VALUE / ß F/ \\ A B C SHEARING STRESS (DYNES/CM 2) Figure 6. Yield values of a Carbopol 934 gel
448 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The utilization of these yield values can be of great importance in evalua- ting drag, greasiness, and slip. It should be pointed out, however, that from a practical point of view, the Bingham Value is not unique. The experimental yield value is actually the point at which the flow or structural breakdown starts and it is the value of most concern in formulating. Thixotropy This phenomenon is observed to occur preferentially, although not exclu- sively in systems with elongated fiat or long particles. For example, it can be observed in a freshly shaken suspension of iron oxide to which a little electro- lyte has been added. Initially, this system behaves as a Newtonian liquid, but in the course of time, the system becomes plastic or gel-like, with a con- tinuously increasing yield value. Here the particles adhere to each other, but the adhesion is so weak that it is completely destroyed by shaking. However, they reconstitute to form their plastic or gel-like state on standing, retaining enmeshed intermicellular liquid. Martin (14) has indicated that the ideal suspending agent should have a high viscosity at negligible shear (shelf life) and a low viscosity at high shear- ing rates (free flowing during agitation). The simple system detailed above meets this description. Electrolytic concentration and valency can effect the time of solidification or gel formation for a thixotropic system. Both tend to increase the time for solidification. Increasing the H + ion concentration, or lowering the pH, increases the time for solidification. A drop of less than a unit in pH in an iron oxide sus- pension changed the solidification time 100-fold. In this same system, raising the temperature resulted in a shortening of the time for solidification. Dilatancy Dilatancy may be considered as the reverse of thixotropy that is, lipophilic systems exhibit low resistance at low shearing rate but high resistance at high shear rates. In these suspensions, there is no permanent contact between par- ticles. Boylan offered the observation that dilitant flow is roughly the opposite of pseudoplastic flow (15). These substances show an increased resistance to flow with an increase in shear. Dilatant materials are highly concentrated systems, usually suspensions. The particles are fine, closely packed, and de- fiocculated. At rest, the particles occupy a minimum volume, with only a very small quantity of liquid filling the interstices. With increasing shear rate, the bulk volume increases and the vehicle is insufficient to fill the void spaces between particles. Interparticle friction increases, resulting in an over- all increased resistance to flow.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)