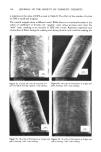
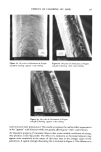
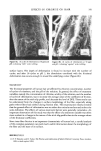
258 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (47) Regulation re preservation of eye cosmetics, 42 Federal Register 54837 (Oct. 1977). (48) U.S. Dept. of Health and Human Services, Public Health Service. Food and Drug Administration, Safe use of eye cosmetics, (FDA, Rockville, Md., 1980), HHS Publ. No. (FDA) 80-5003 ref. 7/80. (49) B. Bhadauria, D. G. Ahearn, Loss of effectiveness of preservative systems of mascaras with age, Appl. Environ. Microbiol., 39, 665-667 (1980). (50) E. V. Platia, R. G. Michaels, W. R. Green, Eye cosmetioinduced conjunctival pigmentation, Annals of Ophthal., 10, 501-504 (1978). (51) C. R. Stewart, Conjunctival absorption of pigment from eye make-up, Amer. J. Optom., 50, 571-574 (1973). (52) J. H.Jervey, Mascara pigmentation of the conjunctiva, Arch. Ophthalmol., 81,124-125 (1969). (53) W. H. Forman, R. V. McDowell, J. A. Shivers, J. R. Steele, Cosmetic eye shadow mimicking orbital calcification (letter to the editor),JAMA, 2695 (1977).
j. Soc. Cosmet. Chem., 33,259-262 (August 1982) Sun protection factor (SPF): a range-finding technique LYNNE B. HARRISON, Ph.D.,, ALICE V. HEALY, R.N., and NICHOLAS BORYS, B.A. Harrison Research Laboratories, Inc., 1814 Springfield Avenue, Maplewood, NJ 07040. Received May 6, 1982. Synopsis A range-finding Sun Protection Factor (SPF) testing technique has been developed. Using a 25 x 2 cm template instead of the conventional 5 x 10 cm, seven subsites can be irradiated. The technique permits the determination of the SPF value through three expected SPF targets. The theoretical, expected SPF value can thus be bracketed and a determination made of the true SPF value of the formulation. In a blind test of five labelled sunscreens the validity of the technique was demonstrated. Five subjects may be used to determine the expected SPF testing can continue in the conventional manner. INTRODUCTION Cosmetic chemists occasionally formulate a product with a relatively unpredictable Sun Protection Factor (SPF) value. A given formulation may reach a full value higher or lower than its theoretical target. The causes of such alteration of the SPF value include the opacity or physical blocking characteristics of other ingredients, effects of the base (e.g., mineral oil), etc. This paper presents an efficient, cost-effective method to perform a range-finding SPF assay to test for three succeeding SPF values. RATIONALE The FDA Proposed Monograph for OTC Sunscreen Drug Products (1) calls for a 50 cm 2 area to be spread with 100 mg or 100 pl (assuming a specific gravity of 1) to obtain a standard 2 mg/cm 2 test application. The suggested template for such a 50 cm 2 is a 5 x 10 cm template. Under "normal" testing circumstances, such a template is efficient. The Monograph (1) calls for at least three test subsite areas that are at least 1 cm 2 to be irradiated at 25% increment/decrement time intervals. It is customary to use five subsite areas of irradiation, with the subject's unprotected Minimal Erythema Dose (MED) multiplied by the expected SPF at the middle subsite, subsite//2 to be irradiated with 25% less (simulated) sunlight, and subsite//1 to be irradiated with 25% less than subsite //2. Similarly, subsite//4 is irradiated with 25% more (simulated) sunlight than subsite//3, and subsite//5 with 25% more than subsite//4. The subject's '*'protected" MED divided by his/her unprotected MED equals the SPF value. It is usual to have the template 259
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)