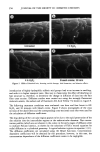
EVALUATION OF HAIR DAMAGE 355 FI (%) lOO 8O 6O 4O 2O Untr I h 4h Treatment Bleach creme Figure 4. Average fluorescence intensity for five each of longitudinally scanned unbleached and bleached fibers treated with Rhodamine B. treatments done in our labs under similar conditions. Although the surface of the hair shows a relatively low level of wettability, we have seen that damage to the fiber interior is more extensive than one might expect from the observed change in surface properties. We also studied the water wettability of unbleached and bleached hair permed one, two, and three times. In contrast to the increased wettability of bleached hair, after bleaching even repeated perm treatments did not produce a major increase in wettability. This observation was somewhat surprising since reductive scission of disulfide bonds leads to the formation of two hydrophilic sulfydryl groups that would be expected to produce an increase in the hydrophilicity of the hair fiber surface. In previous (8) studies of the effect of reduction without subsequent reoxidation (neutralization) on the wettability of hair, we observed that wettability increased rapidly and leveled off at W values of 85-90 mN/m. This difference from the previously observed behavior must be attributed to the fact that the neutralization step (3% H202) following the perming apparently leads to extensive reformation of the original disulfide bonds in the surface regions of the hair. Although bond reformation with peroxide is known not to be complete, the surface sulfydryl groups are the most accessible to reformation and would be the first and most easily affected. Structural changes Dye diffusion. The rate of dye transport in keratin fibers is strongly affected by the nature of the fiber structure. Any decrease in the disulfide crosslink density in the matrix and
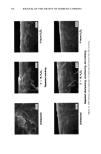
356 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Untreated I h H202 4 h H202 Bleach creme, 30 min Figure 5. SEMs of bleached hair, showing cuticle damage, hole formation, and abrasion effects. introduction of highly hydrophilic sulfonic acid groups lead to an increase in swelling, and with it to higher transport rates. One way to characterize the effect of bleaching on hair structure is, therefore, to determine the change in diffusion of dyes into the hair fiber cross section. Diffusion studies were carried out using the strongly fluorescent molecule uranin, the sodium salt of fluorescein (CI Acid Yellow 73) shown in Figure 8. The following treatment conditions were evaluated: one hour and four hours in 6% H202 and 30 minutes with bleach creme. Figure 9 shows micrographs of the cross sections of the dyed fibers and the corresponding cross-sectional scans that were used for the calculation of diffusion coefficients. The ring dyeing of the cuticular region appears to be due to the rapid penetration of the dye solution into the intercellular ,regions or the endocuticular domains. Dye concen- tration profiles (which are not shown) in the cortex in the early stages of diffusion seem to indicate Fickian kinetics, as shown by the solutions of Fick's second equation for radial diffusion in a cylindrical geometry. A typical solution is given in equation 3 (11). The diffusion coefficients are calculated using the Bessel functions. Concentration- dependent coefficients will be obtained by this procedure however, in this case, the concentration dependence of the diffusion coefficients seems to be negligible.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)