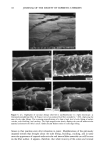
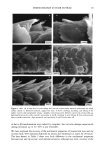
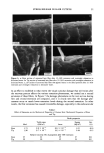
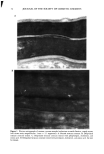
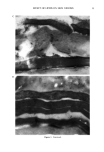
EFFECT OF LIPIDS ON SKIN XEROSIS 29 maintenance of a multilamellar lipid bilayer between the corneocytes has been identified as a key to these benefits (31). Although the skin produces ceramides as the main bilayer-forming lipid in the stratum corneum, we were interested to determine whether other bilayer-forming lipids could mimic their behavior. Phospholipids are the main bilayer-forming lipids found in plasma membranes of living cells. However, they also occur in low levels in the lower layer of the stratum corneum but are usually hydrolyzed in the outer cellular layers. Nevertheless, as they are capable of forming lameliar lipid phases, they may be, there- fore, of use for skin care treatments. We were interested to determine if a mixture of phospholipids, cholesterol, and fatty acids would have beneficial effects in the treatment of skin xerosis, and if these benefits could be enhanced in the presence of glycerol. MATERIALS AND METHODS IN VITRO ELECTRON MICROSCOPY STUDIES Preparation of stratum corneum. Fresh skin (Buckshire Corporation, NJ) was washed with ethanol (70% v/v), cut into 3-cm-wide strips, and dermatomed (0.3-mm thick). The skin was then placed dermis side down onto trypsin solution (0.2% w/v) in sterile, calcium- and magnesium-free, phosphate-buffered saline (PBS), and incubated at 4øC for 18 hours. The epidermis was separated from the dermis and floated epidermis side down on fresh trypsin solution and incubated at 37øC for two hours to release epidermal cells. This procedure was conducted two more times with fresh trypsin and followed by rinsing in PBS. The resulting isolated stratum corneum was floated onto a nylon mesh and desiccated. The isolated stratum corneum was delipidized by extraction in propan- 2-ol (0.1 g SC/100 ml solvent) in a sealed vial at 42øC for one hour and dried. This procedure allowed reproducible extraction of the free intercellular lipids but not the covalently bound stratum corneum lipids. Product treatments. The delipidized stratum corneum was treated with the following three solutions: 1. Chloroform-methanol (2:1 v/v) containing 24 mg/ml of a mixture of phospholipid, cholesterol, and stearic acid (1:2:1 by weight). 2. Chloroform-methanol (2:1 v/v) containing 24 mg/ml of a mixture of petrolatum, cholesterol, and stearic acid (1:2:1 by weight). 3. Chloroform/methanol solution (2:1 v/v). In each case, a known weight of solution was dispensed onto the surface of the stratum comeurn at a loading of approximately 300 I&g/cm 2 and gently rubbed into the stratum corneum. The treated stratum corneum pieces were sandwiched in a nylon mesh to maintain the orientation, and immediately prepared for electron microscopy as described below. Electron microscopy of stratum comeurn. The stratum comeurn samples were pre-fixed in 0.2 M sodium cacodylate-buffered glutaraldehyde solution (2.5% v/v, pH 7.2) for approx- imately 18 hours and then cut into 1-mm-wide strips. The strips were rinsed in cold buffer (0.2 M sodium cacodylate) for 20 minutes followed by a two-hour soak in fresh cold buffer. This step was followed by two further 20-minute rinses in cold buffer. The strips were then post-fixed in ruthenium tetroxide solution (0.2% in 0.2 M sodium
30 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cacodylate buffer) for 15 minutes. The cold buffer rinse and soak procedure above was then repeated. The stratum corneum was dehydrated by soaking in acetone solutions (25%, 50%, 70%, 95%, 100%, 100%, 100%) for 15 minutes each. The stratum corneum was then infiltrated with Spurr resin:acetone solutions (50:50 v/v for 18 hours 70:30 for 3 hours 90:10 for 30 minutes 100:0 for 30 minutes 100:0 for 18 hours). After cutting the stratum corneum from the nylon mesh, the stratum corneum was embedded in fresh Spurr resin and cured at 60øC for approximately 72 hours. Sections were cut (500-600 angstroms), stained with uranyl acetate and lead citrate, and viewed using the JEOL 1200EX electron microscope. IN VIVO DRY SKIN STUDIES Panelist screening criteria. Each study group was comprised of Caucasian women aged over 25, who are susceptible to dry skin and could therefore be expected to show the clearest response to moisturization treatment. None of the panelists took part in any other study for the duration of these studies. All of the panelists were healthy and free of any medical or physiological condition that might affect the assessment of skin or its reaction to the treatment plan. The panelists were not regularly taking drugs or medication and were not nursing or knowingly pregnant. All panelists gave written and witnessed informed consent. Experimental design. These were double-blind, fully randomized clinical trials. All treat- ments and assessments were conducted on the dorsal aspect of the hands. The same protocol was used in each trial and consisted of the following three phases: (1) seven-day "dry-down" (2) 14 days of treatment (3) five days of regression. During the dry-down phase all panelists washed the back of their hands between two and four times a day with soap to induce dryness. The treatment phase consisted of twice- daily product application (am and pm) with continued soap washing (two times per day). During the regression phase, panelists ceased all product application, but contin- ued with the twice-daily soap washing. In addition, all panelists abstained from using any other moisturizer on or near the hand during all phases. In each study group, 75 panelists who underwent the dry-down had their hands visually assessed for dryness on Day 1 of the study, using a seven-point hand dryness scale (see Table I for grading scale). The grading was carried out using an Optivisor © with a number 10 lens (mag. x 3.5) and an Anglepoise © lamp with a 60-watt daylight bulb. The 66 panelists that best met the dryness criterion of grade-5 dryness score on Day 1 were selected to continue with the treatment and regression phases. Each panelist was randomly assigned to a pair of treatments, and one treatment was tested on each hand. The treatments were allocated such that each treatment was tested on 11 hands of 11 panelists, balanced for right and left hands. Treatment products were packed for individual panelist use, in containers fitted with dispensers that delivered 0.5-ml aliquots, with each panelist receiving approximately 100 g of each of the two formulations assigned to her. Panelists were shown how to dispense and apply the product (0.5 ml per application, two times per day). To enable comparison between studies, two control treatments were included in each study. These were an untreated control (negative control) and a standard commer- cial moisturizer (positive control). The skin condition of the backs of the hands was visually assessed as described above on Days 3, 5, 8, 10, and 12 of the treatment phase, and on'Days 15, 17, and 19 of the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)