EVALUATION OF DENTAL ABRASIVES 165 The slurries of reference material calcium pyrophosphate supplied by Monsanto Co. (St. Louis, MO) or of abrasives were prepared using a mass of 10 g of the material and 50 ml of diluent. In the case of thickeners, the slurries were prepared using a mass of 5 g of the material and 50 ml of diluent because of the large volume of the thickener. The diluent was prepared by adding 5 g of carboxymethylcellulose in 50 ml of glycerin heated to 60øC while stirring to obtain a homogenous mixture. Another 50 ml of heated glycerin was added to the mixture, and then 900 ml of distilled water was added. The stirring was continued at room temperature to obtain a clean solution of diluent. Each radioactive slurry was stirred, and three aliquots of 3 ml were pipetted onto separated planchets. These slurries were dried in an oven with air circulation, at 60øC, carefully to avoid cracking in the dried samples. The beta radiation of 1.71 MeV 3•p (with a half life of 14.3 days) of the dried samples was measured using a plastic scintillator detector. Calculation of abrasivehess indices. To calculate the abrasiveness indices, known as RDA, the 32p counting rate obtained for abrasive material was compared to that obtained for the reference material. A score of 100 for calcium pyrophosphate RDA was considered according to an ADA (American Dental Association) committee (6). Correction factors were also applied in this calculation because different abrasives may present distinct self-absorption and backscatterring radiation characteristics. Particle size and microscopy analysis of abrasives. The particle size of the silica and calcium carbonate samples was determined by sedigraphic method and their particle forms were examined using scanning electron microscopy at the Metallurgy Department of the IPEN/CNEN-SP. RESULTS AND DISCUSSION Table I shows RDA values obtained for six samples of silica and three samples of calcium carbonate, together with their particle sizes determined by sedigraphic method. The RDA results for raw materials used as abrasive agents (silica 1 and calcium car- bonate) presented in Table I varied from 136 to 19. The relative standard deviations of these RDA results, in general, varied from 5.9% to 11.8%, showing a good precision in Table I RDA and Particle Size Obtained for Raw Materials, Silica and Calcium Carbonate Silica 1 (abrasive) Silica 2 (thickener) Calcium carbonate Samples A B C D E F G H I RDA_+ s 136 + 8 94-+ 6 85 -+ 10 7 _+ 1 6.6_+ 1.0 5.5 -+ 2.1 54 + 4 24_+ 2 19-+ 2 s r (%) (5.9) (6.4) (11.8) (14.3) (15.2) (38.2) (7.4) (8.3) (10.5) n 7 8 8 8 5 6 8 8 8 Mean diameter (t•m) 4.26 3.21 2.54 1.20 0.31 0.32 3.13 1.77 1.49 RDA _+ s: RDA arithmetic mean values and standard deviation. st: relative standard deviations of the RDA values. n: number of determinations.
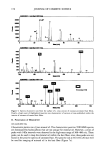
166 JOURNAL OF COSMETIC SCIENCE the method. The silica 2 substances used as agent thickener presented less satisfactory results, with relative standard deviations varying from 14.3% to 38.2% (Table I). The precision of the thickener results was not so good, probably due to the difficulty in obtaining a homogeneous slurry of thickener during the toothbrushing. Table I indicates that there may be a relationship between the RDA results and particle size of each type of raw material, that is, the abrasivity indices of CaCO_• and SiO 2 used as abrasive agents increase with the particle size of the material. However, different types of abrasives with similar particle size presented distinct RDA values. As can be seen in the case of the sample, silica B with a particle size of 3.21 pm presented an RDA value about two times higher than that presented by calcium carbonate G with a 3.13 ]am size. This result is in agreement with those presented by Boer et al. (3). Figure 1 shows the shapes of particles of silica and calcium carbonate obtained by scanning electron microscopy. It can be observed that the particle shapes of the two abrasives are not uniform and that the CaCO 3 appears to have more agglomerated particles than SiO 2. According to Navarre (7), the materials constituted of particles with heterogeneous arrangements and irregular forms are more abrasive than those formed with homogeneous arrangements and regular forms. RDA values for calcium carbonate were also evaluated using different masses of the abrasives in the preparation of slurry with 50 ml of diluent. For 5, 10, 15 and 30 g of CaCO3, the RDA values obtained were 52, 74, 71 and 73, respectively. This preliminary study indicated the increase in RDA values with the mass of CaCO_• until about 10 g. For quantities of CaCO3 higher than 10 g, the RDA values were very close. According to Roa (1), the quantity of CaCO• generally used in dentifrice manufacturing corresponds to about 20 g of CaCO3 in the slurry (50 ml) used in our RDA evaluation. CONCLUSIONS The determination of RDA values of the abrasives can be utilized for prudent selection of raw materials by dentifrice producers. The radiometric method presented here is simple and fast because it does not require long periods for toothbrushing. Also, the RDA results obtained indicated that the abrasiveness of calcium carbonate and silica compounds increased with the particle sizes of the materials. However, it is important Figure 1. A: SiO 2 particles (502x magnification). B: CaCO• particles (447x magnification).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)