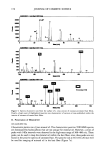
PENETRABILITY OF OILS IN HAIR 181 2O0O00- 0 0 TREATEDP1.TI• + Icx• t211Jm 2082•1 ols l(X)oo TREATEDP1.TDC + • 1211.,m __2n6z•___1 117 137 IL,_ ..L, ............ ,ILl, &, l,•la., t,, ., , .............. 100 120 140 180 180 Ma. [.Vz] TREATEDP1.'1OC + Ions 1211.•n 2082321 ct• .... ...,.i , ........ TREATEI•I.TDC + Io•1 t21pm 20e2•2! Figure 9. Spectra of positive ions from the surface of a cross section of mineral-oil-treated hair fibers. No characteristic positive ions of mineral oil were detected within mineral-oil-treated hair fibers. However, there is contamination from coconut oil and polydimethylsiloxane surfactants. fiber cross sections show little activity, suggesting that the penetration of mineral oil into hair is negligible. The difference seems to be the polarity of the two oils. Coconut oil, being a triglyceride, is polar compared to the nonpolar mineral oil. Therefore, coconut oil has a greater affinity for the cortex of hair, which is also polar in character. III. Effects of Oil Penetration on Swelling Untreated, unaltered hair is known to swell up to 16% in the diametral dimension but
182 JOURNAL OF COSMETIC SCIENCE a b Figure 10. Imaging the characteristic positive ion of mineral oil at mass number 361.26 m/z in the cross section of (a) untreated and (b) mineral-oil-treated hair fibers. No activity was observed, which suggests that mineral oil has not penetrated into the hair fiber interior. only 2% in length upon immersion in water (3,4). This is due to swelling of the globular keratin-associated proteins (KAPs) surrounding the intermediate filament (3,4), as well as of the non-keratinous domains such as the CMC, the endocuticular layer of the cuticle cell, the intermacrofibrillar material, and nuclear remnants (5). The CMC and endocu- ticular domains are known to be the pathways for diffusion of molecules into the hair shaft (5). Oils are known to repel water. Since both the coconut and mineral oils are uniformly coating the hair fiber surface, repulsion of the water molecules upon immersion in water is expected, which, in turn, will inhibit swelling. This is expected to be the case at least during short-term immersion in water. However, during long-term immersion in water, some of the oil molecules may become dislodged by the water, and water molecules will find a passageway into the hair shaft. Since TOF-SIMS clearly identified coconut oil also within the hair fiber cross section, it is expected that the affinity of the protein for the water molecules is reduced, resulting in significantly lower levels of swelling. However, a slightly increased swelling may occur in the case of hair fibers treated with mineral oil because the oil is mainly present on the fiber surface and not in the interior, as estab- lished by TOF-SIMS. To confirm this assumption, untreated hair fibers and fibers treated with coconut and mineral oils were mounted on microscope slides. The fibers were straightened and fastened at both ends, but without tension. Fiber diameters were measured at three marked locations along each fiber. The slides with the fibers were then immersed in DI water in small glass tanks for one hour at ambient temperature. After one horn of immersion in water, the slides with the fibers were removed from the tanks, the bottoms of the slides were blotted, a cover glass was placed on the wet fibers, and the diameters were measured at the same three marked locations along each fiber. The three readings for each fiber were averaged and increases in fiber diameter were calculated. The water-induced swelling observed in untreated fibers was, as expected, significantly reduced in the oil-treated specimens. Figure 11 shows the increase in fiber diameter during immersion in water for each individual fiber and clearly indicates a significant
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)