CONVERSION OF VITAMIN E ACETATE TO VITAMIN E 157 ported by a grid was fitted in the permeation chamber (surface area 28 cm2). Its underside was in contact with degassed PBS, avoiding air bubble formation. The cham- ber was covered with a bell-shaped lid equipped with cocks and enclosing a volume of 46 cm 3, thus permitting the maintenance of controlled conditions during permeation experiments, e.g., to mimic open or occlusion-like conditions. The maintenance of enzymatic activity in the excised skin was monitored as described (13,14). Test solutions of 300 pl were applied to the surface of the mounted skin and evenly spread with a glass spatula. Experiments were carried out under controlled conditions at 23øC and at a maintained humidity of 40%. All experiments were carried out in triplicate and with the skin from three different donors. Experiments were rejected if vitamin Etota 1 was detected in the PBS media ( 100 ng/ml) within 10 min after appli- cation. ASSESSMENT OF VITAMIN E DISTRIBUTION IN SKIN The distribution of both vitamin E acetate and vitamin E was monitored 8 h after application. Vitamin Etota • was collected from the skin surface by wiping with a cotton sponge (Ix dry, lx with 200 pl methanol, l x dry). The horny layer was essentially removed by 20 strippings using adhesive tape (Tesa-Film © [Beiersdorf, Hamburg, Ger- many]), and the remaining skin was frozen and sectioned. Cotton sponges, tape strips, and skin sections were extracted with methanol/phosphate buffer (50/50, v/v). QUANTIFICATION OF VITAMIN E ACETATE AND VITAMIN E BY HIGH-PRESSURE LIQUID CHROMATOGRAPHY (HPLC) Concentrated extracts were separated by HPLC using a reverse-phase column (Luna C 18, 5 pm, 150-mm length, 4.6 mm O, Penomenex © [Aschaffenburg, Germany]) and a mobile phase of methanol/acetonitrile (25/75, v/v). Injection volumes were 100 or 250 pl, and the sensitivity of detection was 25-50 ng/ml. RESULTS Four formulations, each containing 2% vitamin E acetate, i.e., dissolved in Mygliol- 812N (EM), solubilized in water (ES), or encapsulated in liposomes (EL) or in Nano- topes TM (ET), were tested. Of each formulation 11 mg/cm-' was applied to the skin, corresponding to 220 pg/cm 2 of vitamin E acetate. The skin was then maintained for 8 h under defined non-occlusive or occlusive conditions, respectively. Then the amount of vitamin E acetate and vitamin E (tocopherol) was determined for the three compart- ments (skin surface, horny layer, underlying skin). In all experiments, the recovery rate of vitamin Etota • (vitamin E acetate + vitamin E) exceeded 90%. The vitamin E acetate preparations contained --2% of vitamin E prior to application. DISTRIBUTION OF VITAMIN EToT^ r UNDER NON-OCCLUSIVE CONDITIONS Under non-occlusive conditions and when applied in the oil phase (EM), 178.3 (+8%, SD = 3) lng/cm 2 vitamin Eto• l was detected on the skin surface and 28.2 (+31%) lng/cm 2
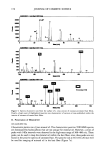
158 JOURNAL OF COSMETIC SCIENCE in the horny layer (more than 80% of it in the first five strips [unpublished result]) only 0.1 (+ 171%) l•g/cm 2 was recovered from the underlying skin. In contrast, when apply- ing ES, 97.2 (+5%) l•g/cm 2 of vitamin Etota I deposited at the skin surface, 46.5 (+8%) lag/cm 2 in the horny layer, and 58.2 (-+9%) lag/cm 2 in the underlying skin. For encap- sulated vitamin E acetate distributed in the horny layer and in the underlying skin, the following amounts were detected. For EL: surface 68.3 (+8%) l•g/cm 2, horny layer 52.6 (+8%) l•g/cm l, underlying skin 79.7 (+3%) l•g/cm 2. For ET: surface 54.4 (+3%) l•g/ cm 2, horny layer 53.2 (+4%) l•g/cm 2, viable skin 97.3 (+4%) l•g/cm l. The relative distribution of vitamin Etota I under non-occlusive conditions is illustrated in Figure 1 and summarized in Table I. DISTRIBUTION OF VITAMIN ET½)TAL UNDER OCCLUSIVE CONDITIONS Under occlusive conditions, with EM, 166.9 (+7%) lag/cm 2 vitamin Etota I was found on the skin surface and 38.2 (+36%) l•g/cm 2 in the horny layer, again concentrated in the first five strips. No vitamin Etota I was detected in the underlying skin. In contrast, after application in ES 108.8 (+5%) lag/cm 2 of vitamin Etota I was localized at the skin surface, 61.9 (+8%) l•g/cm 2 in the horny layer, and 35.7 (+7%) l•g/cm 2 in the underlying skin. A preference of encapsulated vitamin Etota 1 for the horny layer and for the underlying skin was observed also under occlusive conditions, yet to a lesser extent. For EL: surface 91.2 (+7%) l•g/cm 2, horny layer 74.0 (+3%) l•g/cm 2, underlying skin 43.4 (+15%) !ag/cm 2. For ET: surface 65.2 (+7%) l•g/cm 2, horny layer 68.2 (+5%) l•g/cm 2, under- 4- 100 , ! EL EM ES 80 6o 40 ,, ,, ,, ET vitamin E acetate ,:r"•'] free vitamin E Figure 1. Influence of formulation on distribution of vitamin E acetate and vitamin E in human skin. Application on excised human skin under air exchange (non-occlusive conditions). Vitamin E acetate was dissolved in Mygliol (EM), solubilized in water (ES), and encapsulated in liposomes (EL) and in Nano- topes TM (ET).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)