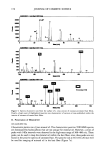
166 JOURNAL OF COSMETIC SCIENCE the method. The silica 2 substances used as agent thickener presented less satisfactory results, with relative standard deviations varying from 14.3% to 38.2% (Table I). The precision of the thickener results was not so good, probably due to the difficulty in obtaining a homogeneous slurry of thickener during the toothbrushing. Table I indicates that there may be a relationship between the RDA results and particle size of each type of raw material, that is, the abrasivity indices of CaCO_• and SiO 2 used as abrasive agents increase with the particle size of the material. However, different types of abrasives with similar particle size presented distinct RDA values. As can be seen in the case of the sample, silica B with a particle size of 3.21 pm presented an RDA value about two times higher than that presented by calcium carbonate G with a 3.13 ]am size. This result is in agreement with those presented by Boer et al. (3). Figure 1 shows the shapes of particles of silica and calcium carbonate obtained by scanning electron microscopy. It can be observed that the particle shapes of the two abrasives are not uniform and that the CaCO 3 appears to have more agglomerated particles than SiO 2. According to Navarre (7), the materials constituted of particles with heterogeneous arrangements and irregular forms are more abrasive than those formed with homogeneous arrangements and regular forms. RDA values for calcium carbonate were also evaluated using different masses of the abrasives in the preparation of slurry with 50 ml of diluent. For 5, 10, 15 and 30 g of CaCO3, the RDA values obtained were 52, 74, 71 and 73, respectively. This preliminary study indicated the increase in RDA values with the mass of CaCO_• until about 10 g. For quantities of CaCO3 higher than 10 g, the RDA values were very close. According to Roa (1), the quantity of CaCO• generally used in dentifrice manufacturing corresponds to about 20 g of CaCO3 in the slurry (50 ml) used in our RDA evaluation. CONCLUSIONS The determination of RDA values of the abrasives can be utilized for prudent selection of raw materials by dentifrice producers. The radiometric method presented here is simple and fast because it does not require long periods for toothbrushing. Also, the RDA results obtained indicated that the abrasiveness of calcium carbonate and silica compounds increased with the particle sizes of the materials. However, it is important Figure 1. A: SiO 2 particles (502x magnification). B: CaCO• particles (447x magnification).
EVALUATION OF DENTAL ABRASIVES 167 to consider that others factors, such as particle hardness, shape, and distribution, can also affect the abrasiveness of the raw materials. ACKNOWLEDGMENTS We acknowledge Colgate Palmolive Ltd. for supplying the raw materials the Monsanto Company for supplying calcium pyrophosphate reference material the Dentistry School and Faculty of Pharmaceutical Sciences of Ribeirgo Preto, University of Sgo Paulo, for tooth samples Dr. J.J. Hefferren from Kansas State University for advice and CNPq and FAPESP for financial support for this project. REFERENCES (1) W. E. P. Roa, Aplicagio e composigio de dentifrfcios, Aerosol Cosmet., 5, 5-14 (1983). (2) H. Panzeri, Personal communication (Dentistry School of Ribeirio Preto, SP, Brazil, 2001). (3) R. Boer, A. S. H. Duinkerke, and J. Arends, Influence of tooth paste particle size and tooth brush stiffness on dentine abrasion in vitro, Caries Res., 19, 232-239 (1985). (4) S. Kinoshita, T. Arai, and R. Uraguchi, Abrasive properties of commonly ua_d dentifrices, Bull. Tokyo Med. Dent. Univ., 26, 225-242 (1979). (5) H. Panzeri, E. H. G. Lara, F. Siessere, and R. M. Marchetti, Availagio de dentifrfcios. 2 a parte: Forma a distribuigio de partfculas abrasivas, Odontd/ogo Moderno, VI, 13-25 (1979). (6) J.j. Hefferren, A laboratory method for assessment of dentifrice abrasivity,J. Dent. Res., 55,563-573 (1976). (7) M. G. Navarre, The Chemistry and Manuj•kcture of Cosmetics (Continental Press, Orlando, Florida, 1975), Vol. III, pp. 445-470.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)