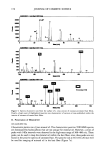
CONVERSION OF VITAMIN E ACETATE TO VITAMIN E 159 Table I Relative Amount (mol %) of Topically Applied Vitamin E .... • Recovered From Different Sites of Human Skin I• Vitro Surface Horny layer Underlying skin Formulation Non-occlusive Occlusive Non-occlusive Occlusive Non-occlusive Occlusive EM Vitamin E ..... 1 79.2 74.2 12.6 17.0 0.4 -- Free vitamin E 1.2 1.2 0.1 0.1 -- -- ES Vitamin E .... • 43.2 48.4 20.7 27.6 26.6 16.6 Free vitamin E 1.3 1.4 0.9 1.3 9.3 8.1 % Conversion 34.8 48.9 EL Vitamin E .... l 30.6 40.6 23.6 33.0 36.8 20.2 Free vitamin E 1.3 1.9 1.3 1.7 14.5 10.5 % Conversion 39.4 51.2 ET Vitamin E .... l 24.5 29.5 24.0 31.1 45.3 31.7 Free vitamin E 1.1 1.5 1.1 1.5 19.5 15.6 % Conversion 43.1 49.2 Topical application of vitamin E acetate onto excised human skin. Vitamin E acetate was dissolved in Mygliol (EM), solubilized in water (ES), and encapsulated in liposomes (EL) and in Nanotopes TM (ET). The amount of vitamin E acetate and vitamin E was quantified by HPLC in the respective extracts. All experiments were carried out in triplicate. lying skin 66.3 (+7%) lag/cm 2. The relative distribution of vitamin Etota 1 under occlu- sive conditions is illustrated in Figure 2 and summarized in Table I. BIOCONVERSION OF VITAMIN E ACETATE TO VITAMIN E The extent of conversion of vitamin E acetate to vitamin E was calculated from the molar ratio of vitamin Etot• • recovered in each compartment as vitamin E, i.e., on the skin surface, in the horny layer, and in the underlying skin. Consequently, the absolute amount of vitamin E depends on both the amount of vitamin Etota 1 transported to a compartment and the local activity of ester hydrolysis. At the skin surface or in the horny layer, the vitamin E determined in all instances essentially corresponded to the less than 2% of the vitamin E contained in the vitamin E acetate formulations prior to application, indicating the absence of relevant esterase activity. In the underlying skin, however, substantial amounts of free vitamin E were detected. If applied as ES, EL, or ET, 26-45% of vitamin Etot• • was recovered from the underlying skin under non-occlusive conditions (Figure 1) and 16-30% under occlusive conditions (Figure 2). Findings are summarized in Table I. Accordingly, the highest absolute concentration of vitamin E in the skin was obtained after application of ET under non-occlusive conditions (ET: 20% EL: 15% ES: 9% Figure 1), as well as under occlusive conditions (ET: 16% EL: 11% ES: 8% Figure 2). The conversion rate of vitamin E acetate to vitamin E, i.e., the molar amount of vitamin E•o• l transformed to vitamin E, was calculated as 35% (ES), 39% (EL), or 43% (ET) under non-occlusive conditions. Though the amount of vitamin E acetate deposited
160 JOURNAL OF COSMETIC SCIENCE 100' + 80 m 60 ._c.• 40 2O EM ES EL ET vitamin E acetate ':• free vitamin E Figure 2. Influence of formulation on distribution of vitamin E acetate and vitamin E in human skin. Application on excised human skin under air occlusive conditions. Vitamin E acetate was dissolved in Mygliol (EM), solubilized in water (ES), and encapsulated in liposomes (EL) and in Nanotopes TM (ET). under occlusive conditions was smaller, conversion amounted to about 50% in each of the three formulations (see Table I). DISCUSSION Vitamin E is known as nature's major lipid-soluble free-radical scavenging antioxidant (4). Due to its instability in the presence of oxygen or light, the use of free vitamin E as a cosmetic active is very limited. Therefore, it has been replaced by stable, oil-soluble precursors, i.e., mainly vitamin E acetate or palmitate. These derivatives are claimed to be converted in the skin to the active vitamin E (4,5). The present study corroborated that vitamin E acetate is a suitable precursor for vitamin E in topical applications. Quantitative analysis demonstrated that vitamin E acetate was only available in the living skin if formulated into a water phase and not in an oil phase. Lipophilic substances are incorporated into the water phase either by means of solubi- lizers or with carrier systems such as liposomes or Nanotopes TM. In the present study, Nanotopes TM deposited more vitamin E acetate in skin than liposomes or the solubilizer under both non-occlusive and occlusive conditions. This consistently better performance of ET may relate to the size of Nanotopes TM, which is about ten times smaller than that of conventional liposomes (12). Bioconversion of vitamin E acetate to vitamin E was localized exclusively in the viable skin (most probably in the epidermal layer [Artmann, unpublished]). Hydrolysis was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)