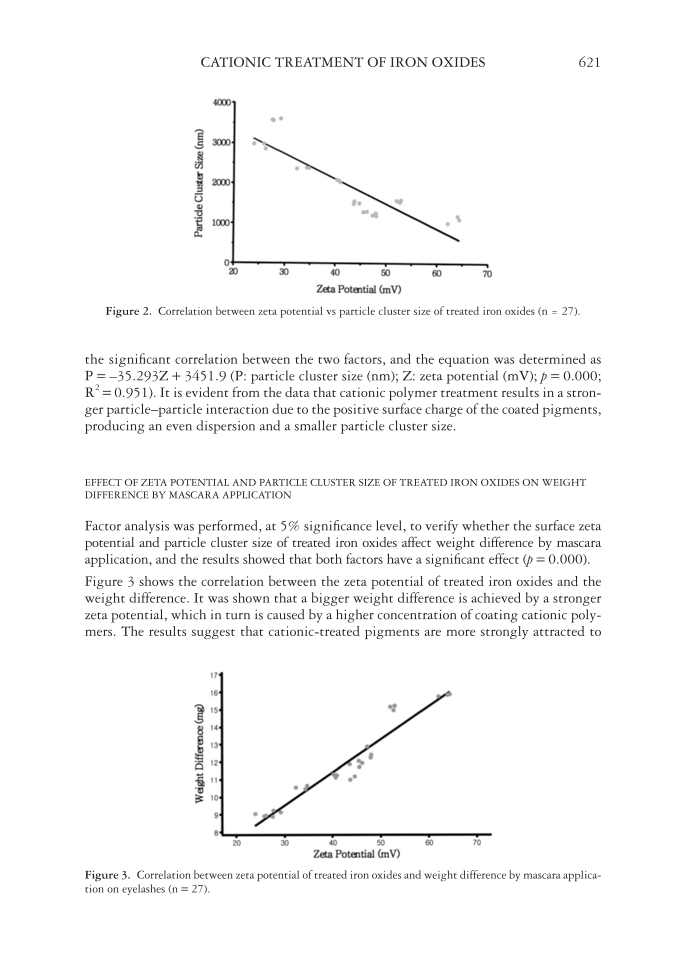
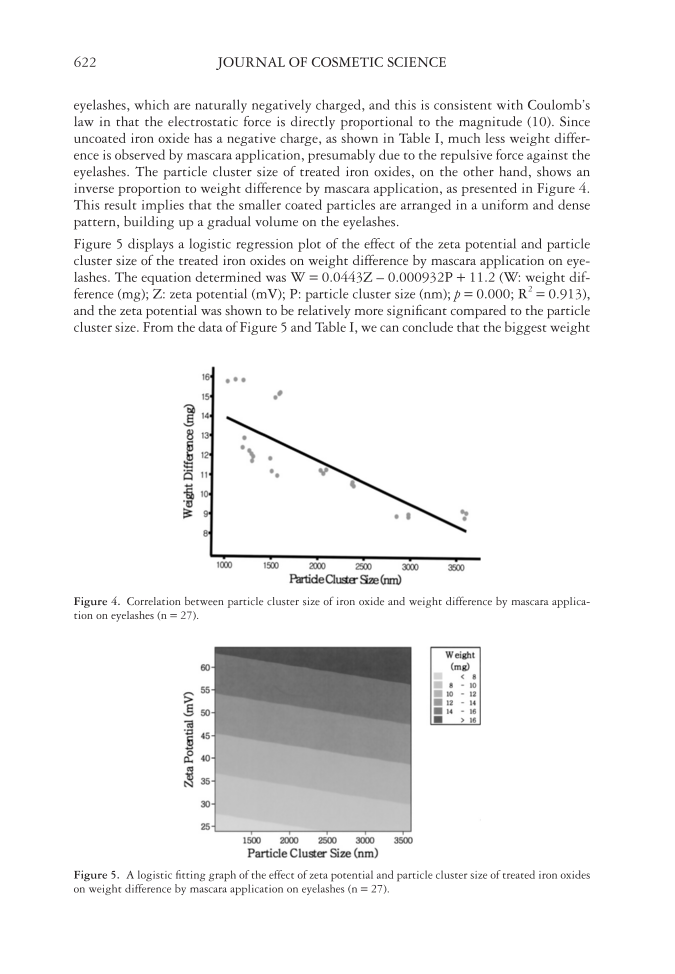
CATIONIC TREATMENT OF IRON OXIDES 621 the signifi cant correlation between the two factors, and the equation was determined as P = –35.293Z + 3451.9 (P: particle cluster size (nm) Z: zeta potential (mV) p = 0.000 R2 = 0.951). It is evident from the data that cationic polymer treatment results in a stron- ger particle–particle interaction due to the positive surface charge of the coated pigments, producing an even dispersion and a smaller particle cluster size. EFFECT OF ZETA POTENTIAL AND PARTICLE CLUSTER SIZE OF TREATED IRON OXIDES ON WEIGHT DIFFERENCE BY MASCARA APPLICATION Factor analysis was performed, at 5% signifi cance level, to verify whether the surface zeta potential and particle cluster size of treated iron oxides affect weight difference by mascara application, and the results showed that both factors have a signifi cant effect (p = 0.000). Figure 3 shows the correlation between the zeta potential of treated iron oxides and the weight difference. It was shown that a bigger weight difference is achieved by a stronger zeta potential, which in turn is caused by a higher concentration of coating cationic poly- mers. The results suggest that cationic-treated pigments are more strongly attracted to Figure 2. Correlation between zeta potential vs particle cluster size of treated iron oxides (n = 27). Figure 3. Correlation between zeta potential of treated iron oxides and weight difference by mascara applica- tion on eyelashes (n = 27).
JOURNAL OF COSMETIC SCIENCE 622 eyelashes, which are naturally negatively charged, and this is consistent with Coulomb’s law in that the electrostatic force is directly proportional to the magnitude (10). Since uncoated iron oxide has a negative charge, as shown in Table I, much less weight differ- ence is observed by mascara application, presumably due to the repulsive force against the eyelashes. The particle cluster size of treated iron oxides, on the other hand, shows an inverse proportion to weight difference by mascara application, as presented in Figure 4. This result implies that the smaller coated particles are arranged in a uniform and dense pattern, building up a gradual volume on the eyelashes. Figure 5 displays a logistic regression plot of the effect of the zeta potential and particle cluster size of the treated iron oxides on weight difference by mascara application on eye- lashes. The equation determined was W = 0.0443Z – 0.000932P + 11.2 (W: weight dif- ference (mg) Z: zeta potential (mV) P: particle cluster size (nm) p = 0.000 R2 = 0.913), and the zeta potential was shown to be relatively more signifi cant compared to the particle cluster size. From the data of Figure 5 and Table I, we can conclude that the biggest weight Figure 4. Correlation between particle cluster size of iron oxide and weight difference by mascara applica- tion on eyelashes (n = 27). Figure 5. A logistic fi tting graph of the effect of zeta potential and particle cluster size of treated iron oxides on weight difference by mascara application on eyelashes (n = 27).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)