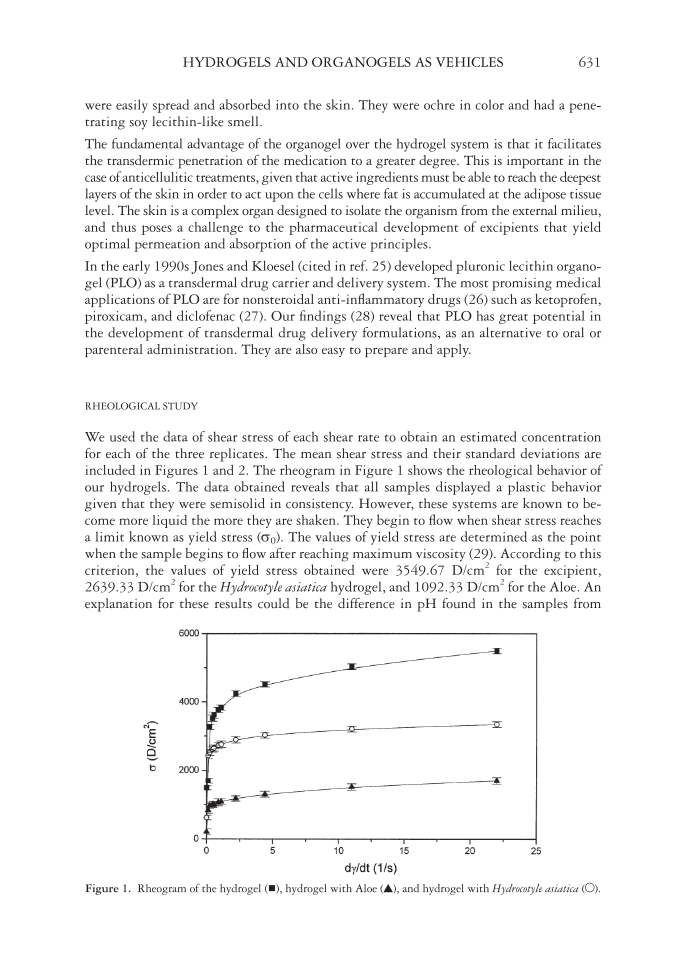
HYDROGELS AND ORGANOGELS AS VEHICLES 631 were easily spread and absorbed into the skin. They were ochre in color and had a pene- trating soy lecithin-like smell. The fundamental advantage of the organogel over the hydrogel system is that it facilitates the transdermic penetration of the medication to a greater degree. This is important in the case of anticellulitic treatments, given that active ingredients must be able to reach the deepest layers of the skin in order to act upon the cells where fat is accumulated at the adipose tissue level. The skin is a complex organ designed to isolate the organism from the external milieu, and thus poses a challenge to the pharmaceutical development of excipients that yield optimal permeation and absorption of the active principles. In the early 1990s Jones and Kloesel (cited in ref. 25) developed pluronic lecithin organo- gel (PLO) as a transdermal drug carrier and delivery system. The most promising medical applications of PLO are for nonsteroidal anti-infl ammatory drugs (26) such as ketoprofen, piroxicam, and diclofenac (27). Our fi ndings (28) reveal that PLO has great potential in the development of transdermal drug delivery formulations, as an alternative to oral or parenteral administration. They are also easy to prepare and apply. RHEOLOGICAL STUDY We used the data of shear stress of each shear rate to obtain an estimated concentration for each of the three replicates. The mean shear stress and their standard deviations are included in Figures 1 and 2. The rheogram in Figure 1 shows the rheological behavior of our hydrogels. The data obtained reveals that all samples displayed a plastic behavior given that they were semisolid in consistency. However, these systems are known to be- come more liquid the more they are shaken. They begin to fl ow when shear stress reaches a limit known as yield stress (σ0). The values of yield stress are determined as the point when the sample begins to fl ow after reaching maximum viscosity (29). According to this criterion, the values of yield stress obtained were 3549.67 D/cm2 for the excipient, 2639.33 D/cm2 for the Hydrocotyle asiatica hydrogel, and 1092.33 D/cm2 for the Aloe. An explanation for these results could be the difference in pH found in the samples from Figure 1. Rheogram of the hydrogel ( ), hydrogel with Aloe (▲), and hydrogel with Hydrocotyle asiatica (○).
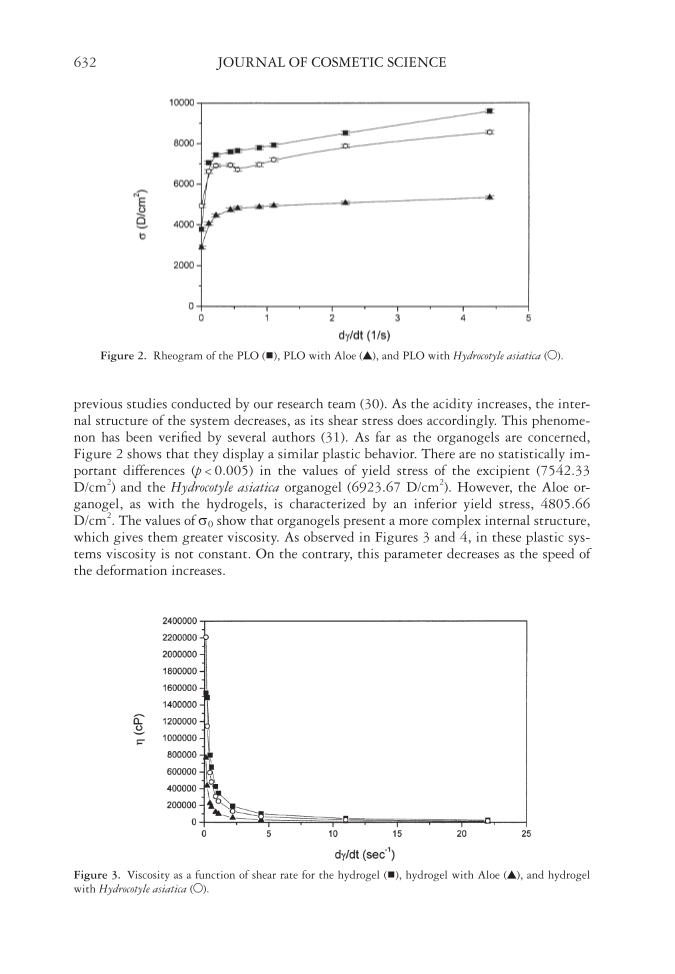
JOURNAL OF COSMETIC SCIENCE 632 previous studies conducted by our research team (30). As the acidity increases, the inter- nal structure of the system decreases, as its shear stress does accordingly. This phenome- non has been verifi ed by several authors (31). As far as the organogels are concerned, Figure 2 shows that they display a similar plastic behavior. There are no statistically im- portant differences (p 0.005) in the values of yield stress of the excipient (7542.33 D/cm2) and the Hydrocotyle asiatica organogel (6923.67 D/cm2). However, the Aloe or- ganogel, as with the hydrogels, is characterized by an inferior yield stress, 4805.66 D/cm2. The values of σ0 show that organogels present a more complex internal structure, which gives them greater viscosity. As observed in Figures 3 and 4, in these plastic sys- tems viscosity is not constant. On the contrary, this parameter decreases as the speed of the deformation increases. Figure 2. Rheogram of the PLO ( ), PLO with Aloe (▲), and PLO with Hydrocotyle asiatica (○). Figure 3. Viscosity as a function of shear rate for the hydrogel ( ), hydrogel with Aloe (▲), and hydrogel with Hydrocotyle asiatica (○).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)