PURPLE SWEET POTATO EXTRACTS IN UV PROTECTION 335 where ApH1.0 = measured absorbance in pH 1.0 potassium chloride buffer with HCl ApH4.5 = measured absorbance in pH 4.5 sodium acetate buffer with HCl DF = the dilu- tion factor MW = the molecular weight of cyanidin-3-glucoside, 449.2 ε = molar ab- sorptivity, 26,900 and d = the optical path of the cuvette (1 cm). Based on the ingredients of commercial sunscreens collected from the market, a plain cosmetic cream recipe was designed for this study to test the UV-absorbing ability of purple sweet potato extract. The cosmetic cream contained 5% emulsifi er, 5% Tween 80, 2% olive oil, 2% xanthan gum, 0.2% disodium EDTA, 1% sodium chloride, 0.2% meth- ylparaben, 0.2% butylparaben, and distilled water. Then 5 or 10 ml of purple sweet po- tato extracts were added per 100 g of cream. The UV-visible spectra of anthocyanin extracts were analyzed using a JascoR V-530 UV/VIS spectrophotometer. Acidic ethanol solution (0.1N HCl (aq) in ethanol, 15:85 (v/v)), was used to dilute the samples before running spectrophotometric analysis. To study the infl uence of pH, 0.1 ml of anthocya- nin extracted solution was dissolved in 40 ml of distilled water. The pH of the solution was increased from acidic to basic by slowly adding 0.1N NaOH(aq). Samples with pH ranging between 4 and 11 were used for UV absorption analysis. The scavenging activity of anthocyanin extracts on DPPH (1,1-diphenyl- 2-picrylhydra- zyl) radicals was determined using the method provided by Huang et al. (28). The radical scavenging activity of ascorbic acid was measured as a positive control. Fifty microliters of the extract was mixed with 150 μl of freshly prepared 1 mM DPPH in ethanol. The mixture was kept in the dark for 30 min. The absorbance of the mixture at 517 nm was then measured using an ELISA reader (TECANR, Austria). The radical scavenging activ- ity was calculated as follows: (1- (ASample/ABlank)) × 100. The EC50 value was defi ned as the effective concentration at which 50% of the DPPH radicals were scavenged, and was determined by interpolation based on linear regression analysis. Total phenolic content was determined according to the Folin–Ciocalteu method, using gallic acid as a standard (29). The extracted sample was dissolved in methanol/water (50/50 (v/v)). Six hundred microliters of the dissolved sample was mixed with 600 μl of 1N Folin–Ciocalteu reagent. The mixture was allowed to stand for 5 min, and then 1 ml of 20% Na2CO3 was added. After a 10-min resting period, the mixture was centrifuged for an additional 10 min (6,000g). Following centrifugation, the absorbance of the super- natant was measured at 730 nm, using a UV-Vis spectrophotometer. The total phenolic content was expressed as the gallic acid equivalent (GAE) in μg/ml of the sample. The reducing ability was measured following the method described by Singh and Rajini (30). Two hundred microliters of the extract were mixed with 200 μl of 1% (w/v) K3Fe(CN)6 and 200 μl of 0.2 M phosphate buffer with a pH of 6.6. The mixture was kept at 50°C for 20 min. Two hundred microliters of 10% (w/v) trichloroacetic acid was then added, and the mixture was centrifuged at 3,000 rpm for 10 min. One hundred microli- ters of the supernatant was transferred to a 96-well plate, with each well containing 100 μl of distilled water and 20 μl of 0.1% (w/v) FeCl3 solution. The absorbance of each well was measured using an ELISA reader at a 700-nm wavelength. To study the infl uence of anthocyanins on the UV protection ability, anthocyanin extracts were added to the prepared cosmetic cream at ratios of 0, 5, and 10 ml/100 g of cream, respectively. After each sample was well-mixed, 0.1 g of the cream was dissolved in 2 ml of acidic ethanol (0.1 N HCl(aq) in ethanol, 15:85 (v/v)). The UV absorbance of the solu- tion was measured using a UV spectrophotometer at wavelengths of 300, 325, 350, and
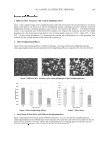
JOURNAL OF COSMETIC SCIENCE 336 375 nm. The prepared cosmetic cream was also evenly spread on a slide. Two cover slides were placed on the ends of the above slide, and another slide was placed on top of the two cover slides, creating a space with a depth of 0.15 mm, equivalent to the thickness of the spread cream layer. The slides fi lled with cream were placed under a UV light source (Model# PS-160, Horng & Fung Corporation, Taiwan). A UV light meter (Model# UV- 340, LutronR, Taiwan) was put beneath the slides to measure the UV irradiation through the samples. A blank test was performed to calibrate the effect of the slides on the UV irradiation. All experimental tests were repeated three times. Data were analyzed using analysis of variance (ANOVA), and the results were compared using the Student t-test. A difference was considered to be statistically signifi cant when the p-value was less than 0.05 ( p 0.05). RESULTS AND DISCUSSION The absorbance of ethanol extract diluted 15× in pH 1.0 and pH 4.5 buffers was 0.686 and 0.198, respectively. Based on Equation 1, the total anthocyanin content was calcu- lated to be 122.2 mg/l. The color of the ethanol-extracted anthocyanin solution was reddish-purple with a pH of 4.1. When the pH was raised from 4 to 11 by the gradual addition of 0.1 N NaOH(aq), the solution color changed from pink to green, and fi nally to yellow. The pH value had a signifi cant effect on the color of the anthocyanin solution. A pH between 4 and 5 was considered ideal, because anthocyanins are more stable under an acidic environment. According to Figure 1, the purple sweet potato extracts demon- strated a good ability to absorb ultraviolet radiation, particularly at wavelengths between 250 and 350 nm. Therefore, the purple sweet potato extracts appear to be an ideal ingre- dient for improving the cosmetic cream’s UV-A and UV-B absorption properties. The results shown in Figure 1 were obtained from solutions diluted 400×. After correcting for the dilution, the UV absorbing ability of the initial extracts was excellent. Although a neutral pH condition resulted in the best UV-absorbing ability, the greenish-yellow color might not appeal to consumers. Figure 1. The infl uence of pH on the absorbance of anthocyanin solution extracted from TNG73 purple sweet potato (with 400× dilution) measured by the spectrophotometric method.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)