HPTLC DETERMINATION OF KETOCONAZOLE 373 METHOD APPLICATION TO SAMPLE The proposed HPTLC method was successfully applied to the determination of ketocon- azole in the three commercially available shampoos and ketoconazole creams. The densi- tograms of the sample solutions are shown in Figure 4. The contents of ketoconazole in shampoo A, shampoo B, and ketoconazole cream A were analyzed using the procedure described in the Experimental section. The results generated by the HPTLC method and the published spectrophotometric method (12) were compared with those expected by the label claims (Table V). The results obtained were compared for agreement with those obtained using the spectrophotometric method. Statistical analysis of the results by using a t-test showed that the calculated t-values were less than the table list t-value at the 95% confi dence limit (p=0.057). Therefore, these two methods are not signifi cantly different in any of the samples. CONCLUSION The proposed HPTLC procedure can be used for the determination of ketoconazole in commercial shampoos and pharmaceuticals. The detection limit of this method was rea- sonably accepted. Sample pretreatment is not necessary. This method is simple, fast, rel- atively inexpensive, precise, accurate, sensitive, and uses a minimum number of reagents and chemicals. Therefore, the speed of analysis and the precision make this method suit- able for quality control of ketoconazole in commercial shampoo and pharmaceutical for- mulations. It is therefore suitable for quality control in drug industries. Table IV Recovery Studies on Ketoconazole Ketoconazole in sample Concentration in sample (mg/ml)* % Recovery* (mean ± SD) Added Found Shampoos: Nora 5 4.91 ± 0.13 98.27 ± 2.52 10 9.86 ± 0.35 98.58 ± 3.47 Kenalyn 5 4.71 ± 0.26 98.09 ± 1.73 10 9.21 ± 0.23 96.04 ± 1.15 Nizoral 5 5.03 ± 0.40 100.59 ± 7.93 10 9.86 ± 0.35 98.58 ± 3.47 Creams: Nizoral 5 4.54 ± 0.43 96.91 ± 2.86 10 9.33 ± 0.31 99.63 ± 1.57 Fungasin 5 4.95 ± 0.18 99.03 ± 3.67 10 9.55 ± 0.34 95.49 ± 3.45 Ketazon 5 4.97 ± 0.49 99.80 ± 3.27 10 9.33 ± 0.31 96.63 ± 1.57 * Mean of three determinations.
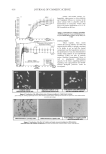
JOURNAL OF COSMETIC SCIENCE 374 Table V Comparison of HPTLC and Spectrophotometric Methods for the Determination of Ketoconazole in Shampoo and Pharmaceutical Formulations Amounts of ketoconazole found % found ± SD* (% labeled amount) Sample Labeled content (% w/w) HPTLC method Spectrophotometric method (12) Nora 2 2.23 ± 0.13 2.35 ± 0.45 (111.50%) (117.50%) Kenalyn 2 2.27 ± 0.17 2.42 ± 0.28 (113.50%) (121.00%) Ketazon 2 2.24 ± 0.27 2.37 ± 0.39 (112.00%) (118.50%) Paired Student’s t-test P value = 0.057 * Mean of three determinations. Figure 4. Densitogram of ketoconazole sample on silica gel 60 F254 in the solvent system, ethanol- acetone- 1.0 mol/l H2SO4 (80:10:10).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)