J. Cosmet. Sci., 61, 367–376 (September/October 2010) 367 High-performance thin-layer chromatographic determination of ketoconazole in pharmaceutical formulations SUWANNA SAYSIN, BOONSOM LIAWRUANGRATH, and SAISUNEE LIAWRUANGRATH, Department of Pharmaceutical Science, Faculty of Pharmacy (S.S., B.L.) and Department of Chemistry, Center for Innovation in Chemistry, and Institute for Science and Technology Research and Development, Faculty of Science (S.L.), Chiang Mai University, Chiang Mai 50200, Thailand. Accepted for publication June 30, 2010. Synopsis A high-performance thin-layer chromatographic method was developed for the determination of ketoconazole. The sample was separated on a silica gel 60 F254 plate and developed in ethanol-acetone-1.0 mol l-1 H2SO4 by means of an automatic multiple-development system. The area of the spot was quantifi ed by a TLC scanner at a wavelength of 298 nm. A linear calibration curve was established over the range of 3–20 mg/ml of ketocon- azole, with a correlation coeffi cient of 0.9992. The relative standard deviations for intraday and interday preci- sions, for three replicate determinations, were found to be 1.72% and 0.69% for 5 mg/ml and 2.18% and 0.94% for 10 mg/ml of ketoconazole, respectively. The average percentage recoveries of ketoconazole shampoos (Nora, Kenalyn, and Nizoral) and ketoconazole creams (Nizoral, Fungasin, and Ketazon) were found to be 96.10, 97.06, and 99.58, and 96.77, 97.26, and 95.74, respectively. This method has been applied to the de- termination of ketoconazole in various pharmaceutical dosage forms. Common excipients in formulations do not interfere. This method is simple, precise, accurate, and inexpensive. It should be used for routine analysis. INTRODUCTION Ketoconazole (cis-1-acetyl-4-[4-2-(2,4-di-chlorophenyl)-2-(1H-imidazole-1-yl methyl- 1,3-dioxolan-4-yl] methoxy piperazine) is an imidazole antifungal agent (Figure 1) (1). As with other imidazoles, it has a fi ve-membered ring structure containing two nitrogen atoms. Ketoconazole is available in many drug dosage treatment forms: cream, sham- poo, solution, gel/jelly, and foam. The side effects of ketoconazole treatment products are rash, itching, nausea, vomiting, abdominal pain, headache, dizziness, fatigue, im- potence, and blood count abnormalities (1). Rarely do the treatment products of keto- conazole have serious allergic reactions (anaphylaxis). Ketoconazole 2% shampoo is used to treat dandruff and it is used to treat “sun fungus” (4–5). Ketoconazole sham- poos may cause abnormal hair texture, scalp pustules (pimples), dry skin, and itching. There may also be oiliness and dryness of the hair and scalp. Rarely, there may be some Address all correspondence to Boonsom Liawruangrath.
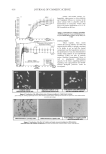
JOURNAL OF COSMETIC SCIENCE 368 hair loss. Ketoconazole is the only member of the immidazole class that is currently used for treatment of systemic infections. Ketoconazole was more widely used before the development of newer, less toxic, and more effective triazole compounds, fl uconazole and itraconazole, and its use has now been limited. It now appears as an alternative drug for specifi c indications. Ketoconazole works principally by inhibition of cytochrome P450 14a-demethylase (P45014DM). This enzyme is in the sterol biosynthesis pathway that leads from lanosterol to ergosterol (2). The affi nity of ketoconazole for fungal cell mem- branes is less than that of fl uconazole and itraconazole. Ketoconazole has thus more po- tential to affect mammalian cell membranes and to induce toxicity (3). The offi cial method normally involves titration in non-aqueous solvent (4–5). Various analytical methods have been reported for the determination of ketoconazole. They are spectrophotometric methods (6–11,13,14), spectrofl uorimetric methods (12), high-per- formance liquid chromatography (15–28), the stripping voltammetric and polarographic method (24), capillary zone electrophoresis (30), and high-performance thin-layer chro- matography (31,32). Very few high-performance thin-layer chromatographic methods (HPTLC) have been reported in the literature and there is no report on the determination of this drug in shampoo formulations. It has the advantages of being sensitive, selective, rapid, accurate and reproducible. The HPTLC method can be used for identifi cation and for control of batch-to-batch consistency in the stability testing of drugs and for purposes of control throughout the entire manufacturing process of drugs, as well as for quality control of the fi nished product. This communication describes the development of the HPTLC method and its applica- tions to the quantitative analysis of ketoconazole in pharmaceutical treatment products such as anti-dandruff shampoos and creams. EXPERIMENTAL REAGENTS AND CHEMICALS Ketoconazole (reference standard assay ³ 98%, Sigma, Switzerland), ethanol, acetone, and ethyl acetate (BDH Laboratory Supplies, England), glacial acetic acid, hydrochloric acid, and sulfuric acid (Farmitalia Carlo Erba, Italy) were used. Ethanol-acetone-1.0 mol/l H2SO4 (80:10:10, v/v/v) was the solvent used for TLC development. The preparation of standard and sample solutions was as follows: Preparation of standard solutions. The required quantities of the ketoconazole standard were accurately weighed and dissolved in ethanol to a fi nal concentration of 1000 mg/ml. Figure 1. Structure of ketoconazole.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)