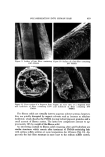
410 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 2O 10 / / / IO 20 30 % ADD ON Figure 1. Add-on and its relation to the amount of PMMA isolated 3. Water rinse 4. Diffusion of oxidizing agent (CHP) into the fibers 5. Reaction between reduced hair and CHP 6. Diffusion of vinyl monomer (MMA) into the fibers 7. Chain-initiating reactions 8. Chain-propagation reactions 9. Termination of free radical chain Influence of Steps I, 2, and 3 on the Overall Reaction Figure 2 depicts the results of experiments showing add-on versus reduction time using 6% TGA at pH 9.2 as the reducing system. Figure 2 also describes data where sodium bisulfite was used as the reducing agent. These results are similar to the data of Wolfram (4), who used a trihydroxymethyl phosphine- persulfate system, and show increasing add-on with increasing time of re- duction.
POLYMERIZATION INTO HUMAN HAIR 411 Z 0 3O 2O I0 I I I I I TGA 5 I0 15 20 25 Na H SO• I 30 t (MINUTES) Figure 2. Influence of the reduction step on add-on These data also show a faster initial rate of polymerization with the TGA system than with the bisulfite system, consistent with the faster rate of reduc- tion of disulfide bonds in hair by TGA. The reaction of TGA with human hair has been shown to be diffusion-controlled (5). Therefore, these data show the importance of step 1, i.e., diffusion of the reducing agent into the fibers, to the overall polymerization reaction. Step 2, the chemical reaction of the disulfide bond with TGA is faster than diffusion and, therfore, of lesser importance to the kinetic scheme however, the extent of this disulfide scission is the prime factor that controls the rate of penetration of monomer and initiator into the fibers, and controls the amount of polymer add-on as shown in Fig. 2 and in the scanning electron micrographs.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)