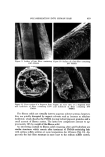
420 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ::.• •: .•, . . :• . ..•. •, .::!•.•v•..•. • . ... ".'.'•.•. : - ...... ':"'" ' .... :-..'½-..:-% •:• '..:...:. '...• . _•.:'•..'•. ,. ..f?..•:t•-•:• •: ..," .: ...? ..:" .:•:...•. ..... :'::"•.':,• .:. •f...•..:..•..:.'. •..•..: •- '.::. ..... .• .:: ..•-,.:•. .• ..:.•2•:'.. --•:: . -• . . •4•.'::..-.. : -- : .... ..•2.., •.. ,..:• .......: ..: . •., .• .-.. ,•....., .. •.•.• •:g•: • ..:' .•..'.::.?•.• .:., :.•. :.: ..:. •.•.?'• . •7•.•'.':.:'•,-.:....:..-.:...•:. . :."-:?• • •::' .•'•:•-• '•': ,'•'::• •'•"'"'...•'•.. •: "4:-:• • •.:..' ..... "•• ' :,: • •.-- • -'- ":.•' ':..•:.•: •?• '..'..' .. •.d' , • .¾•.:-. .:.•...,:%..-.'..•., .•. • ':•..:• ..:•.-?::-'}::..•?• ::.. :...x '•. • •..• • .• •".-::.,•:.•'.'"'.•.. •?' :.::' .. •':... '•.'... ::•.....:,•:• . ... ,. • ..,.,. •...• •-: :'•"::.:.:•? ' :..:'•: .• '••'. • :., ':':. . '. •. • -•-:•..- --:•?: ..... ß .... ..• .•. .i:.•. '•-•: •" ..•...":":..:..:.. ,..• -• .i.. .. . :• :::•: '.... '•. -.• Figure 13. End view of a fragment from sodium sulfide reaction with hair fibers containing 119% PMMA add-on since treatment of fibrous solid remaining from this procedure with benzene or ethylene dichloride results in a relatively slow rate of dissolution leaving behind a much larger quantity of solid matter. Thioglycolic acid-CHP and bisulfite-CHP are effective systems for poly- merizing methyl methacrylate into human hair. The polymer forms initially at or near the fiber surface and grows inwardly. The rate of polymerization is governed by diffusion of CHP and/or MMA into the fibers. Polymerization occurs more rapidly into bleached or reduced-oxidized hair than into chemi- cally unaltered hair, although degree of polymerization is not significantly affected by these same variables. Porous filament-like fragments consisting __
POLYMERIZATION INTO HUMAN HAIR 421 primarily of PMMA were isolated by dissolving fiber from polymer through acid hydrolysis. Sodium sulfide solutions produced similar fragments, not as porous, containing more hair. The possibility for using related techniques for fiber structural studies exists. ACKNOWLEDGMENT The authors wish to thank Mr. John Facq and Mr. Herbert Ohlmeyer for preparing the scanning electron micrographs. (Received August 22, 1973) (9) (10) (11) (19,) REFERENCES (1) Robbins, C., and Anzuino, G., Ionic reactions of keratin fibers containing synthetic polymer, J. Soc. Cosmet. Chem., 22, 579-88 (1971). (2) Anzuino, G., and Robbins, C., Reactions of metal salts with human hair containing synthetic polymer, Ibid., 22, 179-86 (1971). (3) F]ory, P. J., Principles o[ Polymer Chemistry, Cornell University Press, Ithaca, New York, 1953, pp. 308-14. (4) Wolfram, L. J., Modification of hair by internal deposition of polymers, J. Soc. Cosmet. Chem., 20, 539-53 (1969). (5) Hermann, K. W., Hair keratin reaction, penetration, and swelling in mercaptan solu- tions, Trans. Faraday Soc., 59, 1663-71 (1963). (6) Crank, J., Mathematics o[ Diffusion, Oxford University Press, 1967, p. 71. (7) Kharasch, N., Sullenlure Ions and Sulfinyl Compounds, in Organic Sul[ur Com- pounds, Vol. 1, Pergammon Press, New York, 1961. (8) Irwin, R. S., and Kharasch, N., Derivatives of sulfenic acids. XXXVII. Studies of sulfenyl esters (thioperoxides). Part 5. Thermal decompositions, J. Amer. Chem. Soc., 82, 2502-5 (1959). Robbins, C., and Kelly, C. H., Amino acid composition of human hair, Text. Res. J., 40, 891-6 (1970). Zahn, H., Chemical processes in the bleaching of wool and human hair with hy- drogen peroxide and peroxy acids, ]. So½. Cosmet. Chem., 17, 687-701 (1966). Robbins, C., Chemical aspects of bleaching human hair, Ibid., 9,9,, 339-48 (1971). Zahn, H., and Traumann, K., A method for cystine analysis of wool, Melliand Textilber., 35, 1069-73 (1954).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)