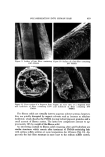
418 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Effect of Previous Treatment of Hair on Molecular Weight Previous Treatment of Hair Prior to Reductive Polymerization Viscosity Ave. Mol. Wt. Unaltered 7.5 to 10.2 x 10 • Bleached 7.6 x 10 • Reduced-oxidized 9.1 x 10 • Polymer Identification and Molecular Weight More extensively bleached hair (once as above with a 24-hour reaction time) provides an add-on of 119% PMMA, using the same conditions of poly- merization. The synthetic polymer in this hair sample was isolated by acid hydrolysis, drying, and extraction with benzene. The residue from benzene extraction was identified as PMMA via infrared spectroscopy and refractive index. The viscosity average molecular weights (3) were estimated in poly- mer isolated in this manner from bleached, reduced-oxidized, and unaltered hair. These data (Table III) suggest that the previous history of the hair, i.e., prior to the reductive polymerization treatment, has little influence on the degree of polymerization of the polymer formed in hair under the conditions of reaction used in this study. Microscopical Examination ol • Isolated Polymer Figures 9 and 10 are copies of photographs taken with a scanning electron microscope (SEM). These photographs reveal the surface of human hair fibers containing a small amount (16%) and a large amount of PMMA (119%). The polymer coating on the fiber surface is thick in both cases, yet the fibers retain some of their surface identity, i.e., repeating irregularities perpendicular to the fiber axis, which correspond to scale edges covered by a thick layer of polymer. Filament-like fragments were isolated from PMMA-containing hair after treatment with 5N hydrochloric acid. These fragments were up to several millimeters in length and roughly the same diameter as the polymer-contain- ing fibers. Microscopical examination, solvent extraction, and infrared spec- troscopy suggest that these fragments consist mainly of PMMA. Figures 11 and 12 are SEM photographs of the fibrous solid remaining after acid hydroly- sis of hair containing 119% PMMA and 16% PMMA. Figure 12 is an end view and Figure 11 a cross section. The sample containing the larger amount of PMMA is a porous yet almost completely filled cylinder while only a thin- walled hollow cylinder remains from the sample with the lower add-on, con- sistent with a diffusion-controlled polymerization process. These results also suggest that the hydrolysis provides a rather selective removal of the hair from the polymer with only minor alterations to the gross structure of the PMMA.
POLYMERIZATION INTO HUMAN HAIR 419 Figure 9. Surface of hair fibers containing Figure 10. Surface of a hair fiber containing 119% PMMA 16% PMMA .,•. ... • -.:-... ..i:-•:•..• ß .•'-.. • - . .- ... .'- ... %- .. . •.. . Figure 11. Cross section of a fragment from Figure 12. End view of a fragment •rom acid hydrolysis of fibers containing 119% acid hydrolysis of fibers containing 16% PMMA I•MMA The fibrous solids are virtually inert to aqueous solvent systems however, they are quickly disrupted by organic solvents such as benzene or ethylene dichloride, which dissolve the PMMA leaving behind pigment granules and a small amount of fibrous matter. The latter two components amount to ap- proximately 10% by weight of the fibrous solid. An interesting contrast to fibrous solid remaining after acid hydrolysis are similar structures which remain after treatment of PMMA-containing hair with sodium sulfide solution at room temperature for 18 hours (Fig. 13). Ap- parently the hair fiber structure is more inert to the sodium sulfide system,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)