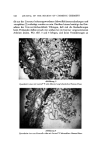
544 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS BLEACHED HAIR 4O E• 30 • 20 ]o !% TERGITOL •' •'"" •" • 0% TERGITOL - ••'"""•-• __2•••___ • i I I I I I I 0 2 4 6 TIME(days) Figure 3. Effect of Tergitol 15-S-9 surfactant on the adsorption of Polymer JR-125 on bleached hair VIRGIN BROWN HAIR TE•5_GI•OL S-9 •08 '• OL • - LAURATE 0 4 -- o• - •• ' TEAL$ and BETAINE-45 0 1 2 3 4 5 SHAMPOO CYCLES Figure 4. Deposition of Polymer JR-400 on brown hair from shampoos containing different surfaetants BLEACHED HAIR • 6 ß BETAINE-45 __•.- ß EO SULFATE i ! t•S I 2 3 4 5 SHAMPOO CYCLES Figure 5. Deposition of Polymer JR-400 on bleached hair from shampoos con- taining different surfactants
ADSORPTION OF POLYMER JR Table I Appearance of Solutions 545 Surfactant/Polymer JR Ratio a Suroeactant 15:1 5:1 3:1 2:1 1:1 1:2 1:4 Standapol AB45 C C C C C C C Standapol ES-2 C SP P P P P P Miranol (pH7) C C P P P P SP F-90 C C C P P SP aPolymer JR concentration constant at 1%: P stands for precipitate P stands for heaviest ppt SP stands for slight ppt C stands for clear. ante of distilled water solutions containing 1 per cent Polymer JR to which different amounts of surfactant had been added. On dilution (ten- or twenty- fold) of the 7 shampoo concentrates themselves with the tap water used for rinsing, only the Miranol and the laurate systems developed visible precipi- tates, the laurate yielding a particularly heavy one. With virgin brown hair (Fig. 4), the deposition of polymer was lowest for the systems containing Standapol T, F-90, and Standapol AB-45 (pH 6). Deposition increased with Standapol ES-2 and more so with potassium lau- rate. The pH of this latter system was adjusted to 10. Miranol (at pH 7.5) and Tergitol 15-S-9 caused the greatest deposition of Polymer JR. With bleached hair, the deposition was about the same for triethanolamine lauryl sulfate, sodium dodecylbenzene sulfonate, ]auryl dimethyl betainc, and the sodium sulfate of the lauryl alcohol ethoxylate all of these gave less than i ./zg of polymer per milligram of hair after 5 cycles of shampooing, and the deposit was scarcely visible by scanning electron microscopy (SEM) (Fig. 6). Considerably more polymer was brought down by Tergitol 15-S-9 and Mira- nol, viz., about 5/zg/mg of hair, and the latter was readily visible by SEM. The greatest deposition occurs with potassium laurate-over 10 /ag/mg. The deposition in this case appears as a nonuniform layer covering the hair scales by the SEM technique (Fig. 7) most likely it consists of precipitated calcium soap together with Polymer JR. This result is of interest in light of the similar deposition on skin found from soap bars with Polymer JR, referred to as fol- lows. Skin The adsorption of Polymer JR on skin shows the same general behavior as on hair: a sharp uptake at short times, followed by a slower process that takes at least a week to reach equilibrium. Fig. 8 shows the experimental data at 0.1 per cent concentration for stratum corneum of fetal pig and calf. On a
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)