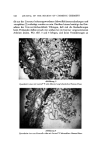
PROPERTIES OF ETHOXYLATED LANOLIN 527 PEG-?5 Lanolin 0tl PEG-75 Lanolin •ax eo•J• 0/%Wt, 1.010 1.020 1,0•0 1.040 1.050 1.0b0 %070 1.0•0 1,090 MOO ß ß • 130 •2.1.'. '..' ........ ' ....... 2• ..'d'•,:. •..•'•' • ....... •. ,.?'. .... J.":." .... 2.'...•':J..: • J'• • ! Laneth-10 Acetate 4 '.. '"'• ' . .' ::•'•'. '..'" '.. ' .... . . i i Figure 2. Specific gravity of lanolin ethoxylates in aqueous solutions on analysis of the results, as the tnolecular xveight of the derivative increases, the cmc decreased as shoxvn in Figure 1, for the lanolin alcohols. In general, we feel that with more accurate methods of cmc determination being avail- able, they nfight be used to obtain a more accurate picture of lanolin ethoxy- lares than the surface tension method presented at this time. In the future, comparisons of methods xvill be evaluated. Figure 2 and Table III illustrate that both specific gravity and viscosity increased as the concentration of ethoxylates in water increased. Thc excep- tion to the rule was found with the PEG-75 Lanolin oil solutions. A mamimum viscosity was obtained at a 50 per cent concentration. Specific gravity values did not follow the expected norm, but showed a maximum value at a concen- tration of about 75 per cent. From the data, it is evident that very slight changes in specific gravity and viscosity were seen within the range of con- centrations use. Further increases in the concentration were not possible be- cause the solubility of most of the lanolin ethoxylates in water at 25øC is lim- ited to 5 to 10 per cent. In ninny cases, at higher levels, the insoluble por- tions floated to the surface, and the lower portion remained a clear gel. It
528 JOURNAL OF THE SOCIETY OF COSMETIC CHE,MISTS Table III Log Viscosity (CPS) of Ethoxylate Solutions to the Limit of Solubility in Water at 68øF Product Concentration Log Viscosity wt % (cPs) PEG-75 Lanolin Oil 100 3.20 75 3.75 50 4.18 25 3.19 20 1.00 10 0.93 5 0.63 i 0.54 PEG-75 Lanolin Wax 45 5.91 40 5.41 35 5.34 30 3.99 25 2.57 20 1.79 10 0.90 5 0.70 I 0.54 Laneth-40 5 0.60 I 0.54 PEG-24 Hydrogenated Lanolin Laneth-16 Laneth-10 Acetate 5 0.60 1 0.54 0.54 0.48 8 0.88 6 0.88 5 0.8O 3 0.80 2 0.70 should be further noted that the low range of viscosity values determined are borderline accuracies per sensitivity of the Brookfield viscometer. The perfume solubilization study demonstrated, that in most cases, the higher level of lanolin ethoxylates produced more significant results. In Table IV, it is shown that Laneth-16 appears to be the most effective solubilizer for these systems. The lanolin alcohol derivatives show superiority to the lanolin ethoxylates as solubilizers. In most cases, there was greater solubility demon- strated at elevated temperatures (105øF). The solubilized perfume com- pounds'. that were evaluated indicated good fragrance qualities. These finished prodrt{.ts all demonstrated an emollient "after-feel" to the skin. Finally, it
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)