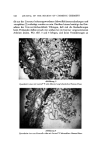
548 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table II Average Skin Dryness Scores Time (days) Group 0 7 14 21 28 1 (0% JR) 1.18 3.31 3.17 4.50 4.67 2 (1% JR) 1.25 2.50 2.99 4.22 4.07 3 (2.5% JR) 1.42 3.10 2.90 3.06 3.21 for such an effect, obtained by applying Polymer JR from soap bars.* The bars, of standard 80/20 tallow/coconut-base coinposition, were made with 0, 1, and 2.5 per cent Polymer JR-400. In a small-scale laboratory test, in which panelists were given a control bar and a bar containing i per cent polymer to use under normal conditions for a week, there was a 2:1 preference in skin "after-feel" for the experimental bar. The perceived improvement in skin feel was correspondingly greater for bars containing 2.5 per cent Polymer JR, and this was confirmed in a larger-scale test involving 100 panelists. For more quantitative assessment of skin condition, the bars with 0, 1, and 2.5 per cent Polymer JR were submitted to Hill Top Research, Inc.* for home use panel testing during the winter of 1974/1975. A panel of 54 women, with a history of wintertime hand chapping, was divided into 3 groups of 18 so as to give an approximate balance of initial hand dryness scores among the test groups. After a week of preconditioning, in which a standard soap and hand lotion were used, the hands were scored for skin condition. Thereafter, the panelists were given the experimental bars, each group having bars of one particular level of Polymer JR, for use at home without any application of lotion. Scores after 0, 7, 14, 21, and 28 days are given in Table II. They were based on skin ratings of 0 through 11, ranging from smooth and soft skin (score 0) through different degrees of flaking, cracking, and fissuring, to se- vere fissuring (score 11). After both 21 and 28 days, group 3, using the 2.5 per cent Polymer JR bar, showed a level of skin dryness significantly (P = 0.05) less than that shown by group 1 using the control bar. Directional trends also indicated an im- movement for the I per cent JR bar over the control, as well as a reduction of fingernail splitting for both polymer-containing bars. Supporting visual evidence for the previous results was obtained by com- paring the skin condition of a female subject, who had washed her hands 3 times during I hour intervals with a commercial soap, and then 3 times there- *Duve•n Co., Brooklyn, N.Y. 11237. '½Miamiville, Ohio.
ADSORPTION OF POLYMER JR 549 after at hourly intervals with a 1 per cent Polymer JR bar. The skin was repli- cated using the technique of Facq et al (12) after completion of the first 3 washings and then again after the second group of 3. SEM revealed a smooth- ing of the skin structure after the second series of 3 washings, as well as the presence of deposited material. CONCLUSION It has been shown that substantial adsorption of Polymer JR occurs on both hair and skin. The size of the uptake far exceeds that expected for simple monolayer adsorption onto the outside surface of these keratin substrates, and indicates that penetration of the polymer into these (porous) substrates does occur. Consequences of such penetration include possible barrier effects against surfactants and moisturization effects. While it is not possible to rationalize fully the observed results on adsorp- tion and deposition in terms of the interaction patterns presented in this paper and in Part I, certain guidelines do emerge. On the one hand, the surfac- rant-polymer systems exhibiting highest deposition on hair, included a "non- interacting" surfactant (Tergitol 15-S-9), as expected, but also the "interact- ing" surfactants potassium laurate and Miranol. The precipitates developed by the latter shampoos on dilution evidently promote adsorption of the poly- mer, and, possibly, the high pH of the soap has an effect. On the other hand, much lower adsorption was .obtained with the other "interacting" surfactants, and this evidently results from the modified nature of the polymer in these solutions (see Part I of this paper). One anomaly, currently under study, is the low uptake observed with the other "noninteracting" surfactant, viz., the betaine. A possible factor in this case is that this surfactant itself adsorbs on the keratin, and in this way influence the adsorption of the polymer. ACKNOWLEDGMENT The electron micrographs were supplied by Dr. Charles Garber of Struc- ture Probe, Inc., 535 East Gay Street, West Chester, Pa. 19380. (Received December 13, 1974) ]REFERENCES (1) F. W. Stone and J. M. Rutherford, U.S. Pat. 3,472,840 (Oct. 14, 1969). (2) J. A. Faucher et al., unpublished communication. (3) G. V. Scott, C. R. Robbins and J. D. Barnhurst, Sorption of quaternary ammonium surfactants by human hair, J. Soc. Cosmet. Chem. 20, 135-52 (1969). (4) J. Woodard, Aziridine Chemistry-applications for cosmetics, J. Soc. Cosmet. Chera., 23, 593-603 (1972). (5) P. Finkelstein and K. Laden, The mechanism of conditioning of hair with alky• quaternary ammonium compounds, Appl. Polltin. Sitrap. No. 18, 673-80 (1971).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)