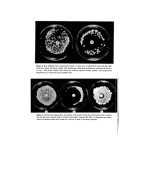
J. Soc. Cosmet. Chem. 28 21-24 (1977) ¸ 1977 Society of Cosmetic Chemists of Great Britain Microbiological applications of a novel replipad skin sampler H. DIXON and A. K. PAN EZAI'• Beecham _Products Research Department, Randalls Road, Leatherhead, Surrey KT22 7RX Synopsis A bacteriological skin sampler which can be used to obtain microbial prints of the axilla is described. Various applications include in vitro and in vivo evaluation of the antimicrobial properties of deodorant and other products using the subject's skin microflora. Introduction The human axillary microflora is of special interest to the cosmetic microbiologist when testing deodorant products, since bacteria have been reported to have association with body odour (1, 2). Various methods have been used for sampling the skin microflora which include contact plate, agar sausage, sticky tape stripping, swabbing, scrub tech- niques and skin biopsy, but because of the peculiar shape of the axillary vault recoveries may be unreliable and few of these methods are suitable in practice. The velvet pad method originates from the well-known replica-plating technique (3) which employs a sterile velvet pad to print bacteria from the master plate onto culture plates containing special media. This method has been extended by other workers and "i'! used for studying the bacterial flora of infected wounds and burns (4), for evaluating the ß 1111'i .relative efficiencies of common skin antiseptics (5), and as a means of making colony : . ':::?::i: counts of pathogenic and commensal flora on the body surface (6). ,: srxN S^m'LER (WLVnT REPL•V^D) "::'i!•.11 The skin sampling device suggested by the authors was developed by *Strands Scientific :•i specially to fit the axillary vault. Bacteria colonizing the skin surface are transferred by •. •:: means of a sterile, moistened velvet pad to a suitable recovery medium. A sufficient :::!':i: number of organisms attach themselves to the velvet pad to enable culture plates to be •::7(.!' successively inoculated. The sampling tool consists essentially of an anodised tapered :::i:11: holder and clamping ring which together hold the circular velvet pad (Fig. 1). When ?:?. assembled, the sampler is enclosed in an autoclavable bag and sterilized in an autoclave ?./• i:.. at 121oc for 15 min. ::.::•.i!i:': Tø obtain a microbial print, the sterile velvet pad is moistened and rendered slightly •!•:.::! sticky, by bringing it into contact with the moist surface of an agar plate (the recovery ? 'i-., Strands Scientific, Scientific House, 32 Bridge Street, Sandiacre, Nottingham NG10 5BA. •'i:•! I::':' • Present address: Bacteriology Dept., Building 320, The Wellcome Foundation, Temple Hill, '!'•:: Dartford, Kent, where requesls for reprints should be sent. 21
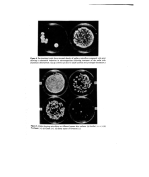
22 H. Dixon and A. K. Panezai medium). The velvet head of the sampler is pressed firmly into the axilla, and then applied to the same area of culture plate. After suitable incubation a microbial print of the axilla surface is obtained. Brain Heart Infusion Agar (B.H.I.A.) supplemented with 0.55/o Tween 80 is recom- mended for the isolation of axillary bacteria. After 24-48 h, the common resident bac- teria, staphylococci, pigmented micrococci and diphtheroids may be readily seen and identified. TESTING A DEODORANT FOR ANTIBACTERIAL ACTIVITY IN VITRO For this purpose a modified agar diffusion test is used. The method of evaluation departs from standard procedure by using freshly isolated resident axillary micro flora as test organisms in place of type culture or stock culture strains which have been passaged several times artificially. For economy, the test is carried out by using small disposable Petri dishes 60 x 13 mm (Sterilin). About 12 ml of B.H.I.A. are poured into each dish. When hardened the agar is inoculated by means of the axilla sampler as previously described. A 6-mm antibiotic assay disc (Whatman) is impregnated with the test deodorant and placed in the centre of the print. Blank discs impregnated with sterile water and a reference standard consisting of the test antimicrobial in simple aqueous solution should be set up at the same time. Dishes are incubated at 37øC for 24 to 48 h after which zones of inhibition are recorded (Fig. 2). INTERPRETATION A deodorant with good antibacterial potential should give a well-defined clear zone of inhibition. Antimicrobial activity is sometimes modified by interaction with other formu- lation ingredients. Such interaction can be dramatically revealed by comparing the activity of th• antimicrobial in aqueous solution with the finished product containing the same active ingredient (Fig. 3). For products which may exhibit antibacterial activity but diffuse poorly, the following direct contact method is recommended. DIRECT CONTACT IN VITRO TEST A sterile filter circle (Whatman 4.25 cm) is treated with test material, drained, and applied to an axilla print for 1 h after which it is removed. Suitable controls should be prepared at the same time consisting of sterile filter circles treated with sterile water, the appropriate solvent and the product without active ingredient. Circles are removed after 1 h as in the test procedure. Plates are incubated at 37øC and presence or absence of growth recorded after 24 and 48 h. EVALUATION OF PRODUCT'S ANTIBACTERIAL ACTIVITY IN VIVO Skin lipids are known to have the effect of greatly reducing the activity of some antimicro- bial agents, so that for realistic results the product needs to be evaluated on the skin, and for deodorants, the axilla is the appropriate area for testing. Before commencing the trial it is important to establish that toiletries which contain antimicrobial agents (anti-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)