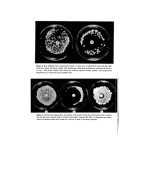
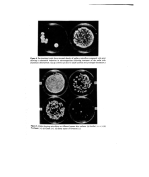
Preservatives for cosmetics and toiletries 5 ./1:1(12, 13, 14, 15) of available preservatives be merely chosen. In fact to the uninitiated these lists can be misleading since some of the agents may not be effective as preservatives •' 'because of their limited antimicrobial spectrum. Examples of these are the quatern- ary ammonium compounds, trichlorocarbanilide, the halogenated salicylanilides and bromochlorophen, all of which are potent antimicrobials as active agents for preparations 'such as soaps and scrubs where activity against Gram-positive bacteria and substan- tivity to the skin are the prime considerations. They are not effective preservatives. Every formulation must be considered on an individual basis although some general rules apply (3). The development of a new cosmetic or toiletry formulation involves consideration and adequate testing of the preservative system at an early stage and the formulatots and the microbiologist should work in close collaboration. The ideal preserva- tive does not exist and no one preservative system is satisfactory under all conditions. It may be that a combination of preservatives has to be used to obtain the desired effect in a particular formulation. The selection of a preservative depends on many factors 05) important considerations are the physical and chemical nature of the product, its potential use (e.g. on normal or broken skin, on infants, around the eyes), the type of container and the desired shelf life. All too frequently the final formulation can only be achieved on the basis of com- .'i:i.i promise safety, compatibility and efficacy are the most important factors influencing the preservative system. As Parker (16) points out although it is essential that the preservative capacity of a cosmetic is adequate to combat spoilage and in-use con- tamination it should not bestow the character of an antiseptic on the preparation. Many dermatologists regard the preservative as a necessary evil. Tronnier (17) makes the plea :. that cosmetic preservative levels should not be such as to upset the equilibrium of the biozone created by the microflora of the skin. The search for new preservatives continues and new ones appear but no single example has yet matched up to the ideal. It has been suggested that it is perhaps unrealistic to expect any compound to possess all the properties attributed to the ideal preservative (3) this belief is supported by the author. PROPERTIES OF THE IDEAL PRESERVATIVE The properties of the ideal preservative have been listed by many workers (e.g. 3, 12, 18). The following factors must be taken into consideration. Antimicrobial activity Any preservative must have broad spectrum antimicrobial activity since Gram-negative and Gram-positive bacteria, fungi and yeasts may be involved as contaminants and/or spoilage agents. The use of a preservative with high activity against some organisms and much lower activity against others may lead to the selection of insensitive organisms. One of the major disadvantages in the use of 2, 4, 4'-trichloro-2'-hydroxydiphenyl ether (lrgasan DP 300) as a preservative is its Pseudomonas gap (19). Since pseudomonads are the most commonly encountered contaminants in aqueous products their importance cannot be overlooked (10, 20). They can render conditions suitable for less adaptable spoilage organisms for example they can create conditions favouring the growth of anaerobes (2).
6 Betty Croshaw Coates (18) has reviewed the microbiological requirements of a preservative. While screening tests in solid or liquid media for the evaluation of the antimicrobial activity of potential preservatives are valuable in selecting agents worthy of further study they will not necessarily predict the activity in a formulation. This is readily understandable as other factors are involved, particularly the o/w partition coefficient since any effective preservative must be present in the aqueous phase. Thus, a compound whose m.i.c. is 1000 [tg/ml may protect an emulsion at a concentration of 0-1-0.2• while another agent which inhibits growth at 20 [tg/ml in screening tests can fail to protect an emulsion even at a level of 0-45/o (21). On the other hand, Charles and Carter (22) found that the parabens, esters of p-hydroxybenzoic acid, were more effective in finished formulations than had been expected from their performance in component testing. Our own work with some formulations supports this a potential preservative must be further evaluated in different formulations at various concentrations to determine its efficacy under dif- ferent conditions. Bactericidal, rather than bacteriostatic, activity is an added advantage although in most cases a preservative may be considered to be effective if it holds small numbers of non-pathogenic organisms in a quiescent state. Even a low level of preservative may be slowly bactericidal and a higher level, resulting in a more rapid bactericidal effect, may be undesirable because of skin irritancy and, indeed, may not be necessary. The number of organisms challenging a preservative system must also be considered and the effect of inoculum size on the activity of any preservative should always be determined in the initial evaluation tests. In a formulated product an overwhelming microbial contamination may swamp the preservative system or, at best, may lengthen the time necessary to reduce the number of organisms to a safe, low level. Resistance studies should also be carried out at an early stage on any potential preservative. Any agent which rapidly selects out insensitive strains of microorganisms is unlikely to be a satisfactory preservative. Toxicological considerations The ideal preservative must be safe in use that is, it must be non-irritant, non-sensitizing and preferably non-poisonous. No chemical can ever be listed as ever being totally non-allergenic or totally non- sensitizing (23). Even the parabens, which have been and still are widely used for the preservation of toiletries and cosmetics, cannot be regarded as completely safe. Paraben allergy was recognized in Europe in the early 1960s and later in the United States when a sensitization index of 0.8• was documented (24). Sorbic acid, which was recommended as a preservative for formulations containing nonionic surface active agents (25) prior to the introduction of some of the newer pre- servatives, has a sensitization index probably slightly lower than that of the parabens (24). The phenolic group of preservatives also has its own problems (24). Two phenolics which are used as preservatives, chlorocresol (p-chloro-m-cresol) and especially chlor- oxylenol (p-chloro-m-xylenol), are known sensitizers and cross sensitization has been reported. The sensitization index for chlorocresol is 0-5•o and in a survey of contact allergy by Calnan (26) chloroxylenol was second only to mercury in terms of sensitivity produced. Formaldehyde, which has been widely used in shaml•oos (5, 7), constitutes a greater
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)