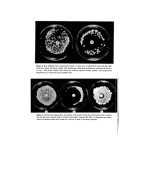
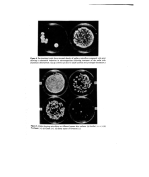
34 A. Meybeck Conclusion The pigments formed by reaction of DHA with aminoacids are very similar to melanins, and like these they can be regarded as highly conjugated polymers bound to exhibit intrinsic E.S.R. properties due to bond alternation defects. They still bear the side chains of the parent aminoacids although decarboxylations occur to some extent with alpha derivatives. The action of DHA on skin must start by condensation with the free aminoacids located on the surface and more probably within the horny cells (23), followed by poly- merization and eventually linking to Stratum Comeurn proteins through the participation of lysine side chains. It seems indeed very unlikely that this polymerization process could occur exclusively with basic aminoacids bound in polypeptide chains and therefore un- able to come close enough to one another. But the fixation of an already grown pigment on protein macromolecules is possible and has similarly been found to occur when wool keratin is immersed in the reaction medium of glycine with glucose (24). Finally, it should be noted that since DHA melanoidins possess the same free spin character as melanins, they too should be able to protect the skin from radiations through trapping of electrons and free radicals which are thought to be important intermediates in sunburn and ageing processes (25). Preliminary investigations of an eventual protective effect of DHA have been negative (26-27) but they should perhaps be resumed in view of these new results and those obtained by Fusaro et al. (28-32) with combinations of DHA and juglone or tawsone. References 1 Von Noorden, C. and Isaac, S. Die Zuckerkrankheit und ihre l•ehandlung 8 Ausg. Julius Springer, Berlin, 415 (1927). 2 Butcher, J., Bandelin, F. and Kanas, N. The reaction of dihydroxyacetone with proteins. Amer. Perf. 75 (12) 46 (1960). 3 Wittgenst½in, E. and Berry, H. K. Reaction of dihydroxyacetone (DHA) with human skin callus and amino compounds. J. Invest. Dermatol. 36 283 (1961). 4 Laden, K. and Zi½linski, R. The reaction of •-hydroxymeth¾1ketones with skin and aminoacids. /. $oc. Cosmet, Chem. 16 777 (1965). 5 Ellis, G. P. The Maillard reaction. Adv. Carbohydrate Chem. 14 63 (1969). 6 Blois, M. S., Zahlan, A. B. and Maling, J. E. Electron spin resonance studies on melanin. Biophys. J. 4 471 (1964) 7 Blois, M. S. On the spectroscopic properties of some natural melanins. J. Invest Dermatol. 47 162 (1966). 8 Mason, H. S., Ingram, D. J. E. and Allen B. The free radical property of melanins. Arch. Biochern. Biooehys. 86 225 (1960). 9 Tollin, G. and Steelink, C. Biological polymers related to catechol: electron paramagnetic resonance and infrared studies of melanin, tannin, lignin, humic acid and hydroquinones. Biochem. Biooehys. Acta. 112 337 (1966). 10 Green, D. B. and Happey, F. The in[rated specta of melanins (natural wool pigments). III e Congr•s oent. de la Recherche Textile Laini•re de France, Paris. I 283 (1965). 11 Charle, R. Pigmentation naturelie et artificielle des cheveux. In: Probl&nes Capillaires. Ed. by E. Sidi and C. Zviak), p. 79. Imprimerie Dermont, Paris (1966). 12 Nechtchein, M. Sur la nature des centres paramagn•tiques d6tect•s par RPE dans les polym•res conjugu6s. J. Pol. Sci.: Part C, 4 1367 (1963). 13 Holob, G. M., Ehrlich, P. and Allendoerfer, R. D. Electron spin resonance in crystallizable, high molecular weight polyphenylacetylene. Macromolecules, 5 569 (1972). 14 Byrd, N. R., Kleist, F. K. and Stareires, D. N. Electric and magnetic properties of polymeric organic semiconductors. J. Pol. Sci.: Part A-2, 10 957 (1972).
Reaction ])roducts of dihydroxyacetone 35 15 16 17 18 19 20 ß 21 24 Huron, J. L. Etude cin6tique des r6actions de d6gradation du polyacrylonitrile entre 200 et 300øC par r6sonance paramagn6tique 61ectronique, chromatographie en phase gazeuse et analyses ther- miques. D.Sc. Thesis, Centre Universitaire du Ht-Rhin, Mulhouse, et Universit6 de Strasbourg, France (1975). Ehrlich, P., Mertzlufft, E. C. and Allendoerfer, R. D. Hyperfine structure in ESR spectra of poly- phenylacetylene solutions. Pol. Letters Ed. 12 125 (1974). Longuet-Higgins, H. C. On the origin of the free radical property of melanins. Arch. Blochem. Biophys. 86 231 (1960). Pullman, A. and Pullman, B. The band structure of melanins. Biochim. Biophys. Acta, 54 384 (1961). Pople, J. A. and Walmsley, S. H. Bond alternation defects in long polyene molecules. Molecular t'hys. 5 15 (1962). Stratton, K. and Pathak, M. A. Photoenhancement of the electron spin resonance signal from melanins. Arch. Biochetn. Biophys. 123 477 (1968). Gottschalk, A. and, Partridge, S. M. Interaction between simple sugars and aminoacids. Nature, 165 684 (1950). Dubourg, J. and Devillers, P. La r•action de Maillard, ses m6faits en sucrerie. Industr. alim. agr. 79 625 (1962). Tabachnick, J. and Labadie, J. H. Studies on the Biochemistry of Epidermis. IV. The free amino- acids, ammonia, urea, and pyrrolidone carboxylic acid content of conventional and germ-free albino guinea pig epidermis. J. Invest. Dermatol. 54 24 (1970). Burton, H. S., McWeeny, D. J., Pandhi, P. N. and Biltcliffe, D. O. Fluorescent compounds and non-enzymatic browning. Nature, 196 948 (1962). Meybeck, A. Electron spin resonance (ESR) in the study of proteins ageing. 8th Int. Fed. Soc. Costa. Chem. Congress, paper D 8, London, 1974. Goldman, L., Barkoff, J., Blaney, D., Nakai, T. and Suskind, R. Investigative studies with the skin colouring agents dihydroxyacetone and glyoxal. J. Invest. Dermatol. 35 161 (1960). Maibach, H. and Kligman, A. M. Dihydroxyacetone: a suntan-simulating agent. Arch. Dermatol. 82 505 (1960). Fusaro, R. M., Runge, W. J., Lynch, E. W. and Watson, C. J. Sunlight protection in normal skin by absorptive filter chemically induced in stratum corneum. Archs Derm. 93 106 (1966). Fusaro, R. M. and Runge, W. J. Sunlight protection in patients with chlorpromazine light sensi- tivity. lnt. J. Dermatol. 10 198 (1971). Fusaro, R. M., Runge, W. J. and Johnson, J. A. Protection against light sensitivity with dihydroxy- acetone/naphtoquinone Int. J. Dermatol. 11 67 (1972). Johnson, J. A., Czaplewski, R. P. and Fusaro, R. M. Protection against long ultraviolet light with dihydroxyacetone/naphtoquinone. Dermatologica, 147 104 (1973). Fusaro, R. M. and Johnson, J. A. Photoprotection of patients sensitive to short and/or long ultra- violet light with dihydroxyacetone/naphtoquinone. Dermatologica, 148 224 (1974).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)