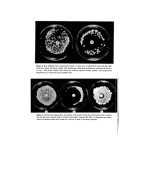
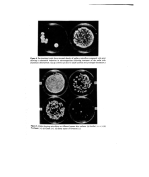
Preservatives for cosmetics and toiletries 7 sensitization hazard than the parabens and the organic mercurials present the greatest hazard (24). Quaternary ammonium compounds are relatively weak sensitizers. The sensitization potential of some of the newer preservatives is not yet fully estab- lished from the clinical point of view. It has been suggested that 2-bromo-2-nitropropane- 1, 3-diol (Bronot•ol) may be used safely at the concentrations normally used in formulations (27). This compound was found to be a mild irritant when applied in soft yellow paraffin on to i49 eczematous patients at a concentration of 0'25% but no evidence of sensitization was seen in this study nor was there any suggestion of cross- sensitization with any other substance particularly formalin. Imidazolidinyl urea (Germall 115) is reported to be non-toxic, non-irritating and non-sensitizing (28) [rgasan DP 300 is said to be free of allergenic or photoallergenic potential on the basis of animal and human tests (29). There is some evidence that 6-acetoxy-2, 4-dimethyl-m- dioxane (Dioxin) is a sensitizer (24). Water solubility The ideal preservative should be readily water soluble at the effective concentration since microbial growth occurs in the aqueous phase. The low water solubility of the organic mercurials, Irgasan DP 300 and the parabens are marked disadvantages. Where water solubility is low, micellar solubilization of preservative molecules by compatible surfactants can be used to increase the amount of preservative in the system (18). Effect of oeH, temt•erature and storage times The pH tolerance limits for microorganisms is between pH 2-11 and since cosmetic and toiletry formulations can cover a wide pH range the ideal preservative should be effective and stable in solution over this range. It should also be stable at temperatures likely to be encountered during the manufacture and storage life of the product. It is well known that only the undissociated molecules of benzoic, dehydroacetic, salicylic and sorbic acids are active against microorganisms and that activity is lost with o , ß increasing pH. The parabens do not have the same pH dependence as benzoic acid but like phenols they show greater activity on the acid side of neutrality. Preservative dissociation as a function of pH has been reviewed by Wedderburn (3). The ideal preservative should be non-volatile at temperatures used during manufac- : ture as well as at normal storage temperatures. Volatility is a disadvantage of chloro- cresol, chloroxylenol and formaldehyde, for example. ß ::::i:: • Effect of oil/water t•artition coefficient .• Since microorganisms multiply in the aqueous phase of formulations the preservative . must be available in an effective concentration in this phase. Aqueous solutions such as :': shampoos are therefore relatively easy to preser•Je since all the preservative is available ::• providing that there is no chemical or physical incompatibility between it and the for- mulation or its container. The situation is obviously more complicated when the for- mulation is a cream or an emulsion as Bean and his colleagues (6, 30, 31) have pointed out the failure of preservatives in this situation may frequently be attributed to the fact that
8 Betty Croshaw only a proportion of the total quantity of preservative is available in the aqueous phase where it is required. The remainder partitions into the oily phase or associates with the emulsifying agent, the other major component, and is thus inactivated. The ideal pre- servative should therefore have a low oil/water partition coefficient. It is therefore desirable that the oil/water partition coefficient (K• ø) of a preservative is known. The concentration in the aqueous phase (Cw) at equilibrium may then be calculated for any total concentration (C) and any oil/water ratio (•) as follows (31): + 1) K•%+l Thus, when the oil/water partition coefficient is low, most of the preservative is in the aqueous phase and an increase in the oil/water ratio in an emulsion increases the aqueous phase concentration. When the oil/water partition coefficient is high most of the pre- servative is in the oil phase and an increase in oil/water ratio reduces the concentration in the aqueous phase (6). High partition coefficient values are usually observed for vegetable oil/water systems and low values for mineral oil/water systems. This means that vegetable oil/water systems usually require a higher total concentration of preservative than do mineral oil/water systems (31). Attention has been drawn to the importance of the concentration of the preservative at the oil/water interface. In an emulsion partition of the preservative occurs between the oil, water and emulsifier and its effectiveness is determined largely but not completely by its concentration free in the water. The distribution of the emulsifier influences the distribution of the preservative. The above equation is modified to: c(o + 1) Cw- R where R is the ratio of total/free preservative in the water (31). Compatibility with other ingredients The ideal preservative should be compatible with all the ingredients of modern cosmetics and toiletries. This is a very tall order in view of the many additives used in present day preparations. Prior to the Second World War, almost all cosmetics and toiletries were stabilized by soaps, which made them alkaline, or by anionic surfactants many anionics have some antibacterial action and also tend to potentiate preservative action (32). Formu- lations containing nonionic surfactants are usually formulated at neutral or slightly acid pH, a factor which enhances microbial growth. Incompatibility with newer cosmetic ingredients and particularly with nonionic surfactants has accounted for the failure of some of the older preservatives such as the parabens which at one time were considered to be ideal agents (3). Barr and Tice (25) and Wedderburn (32) examined the effect of some nonionic surfactants on preservatives in common use at that time and found that many of them, including the parabens, substituted phenols and quaternary ammonium com- pounds were inactivated to some extent when the ratio of the nonionics to preservative exceeded certain critical values. Benzoic and sorbic acids, formaldehyde and organic mercurials were affected to a much less extent. A vast amount of literature has accumu- lated on interactions between nonionic surfactants and preservatives (see reviews 3, 33, 34).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)