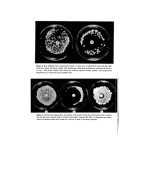
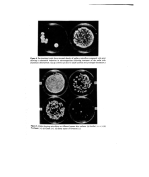
24 H. Dixon and A. K. Panezai The authors wish to acknowledge the helpful advice and criticism offered by Mr N. J. Van Abbd and appreciate the technical assistance given by Mrs P.M. Day and Mr P. J. Edwards. They also wish to thank Mr D. Lawson for his help with the photography. References 1 Shelley, W. B., Hurley, H. J. and Nichols, A.C. Axillary odor Experimental study of the role of bacteria, apocrine sweat and deodorants. Arch. Dermat. and $yoeh. 68 430 (1953). 2 Shehadeh, N.H. and Kligman, A.M. The bacteria responsible for axillary odor II. Jnl. Invest. Dermat. 41 3 (1963). 3 Lederberg, J. and Lederberg, E.M. Replica plating and indirect selection of bacterial mutants. J. Bact. 63 399 (1952). 4 Gorrill, R. H. and Penikett, E. J. K. New methods of studying the bacteria flora of infected open wounds and burns. Lancet, ii 370 (1957). 5 Verdon, P. E. Efficiency tests on a series of common skin antiseptics under ward conditions. o r. clin. Path. 14 91 (1961). 6 Holt, R. J. Pad culture studies on skin surfaces. J. apt•l. oeact. 29 (3) 625 (1966).
J. Soc. Costnet. Chem. 28 25-35 (1977)¸ 1977 Society of Cosmetic Chemists of Great oeritain A spectroscopic study of the reaction products dihydroxyacetone with aminoacids A. MEYBECK Laboratoires Jeanne Gatineau, 27 rue Salvador Allende, 95870 Bezons, France Synopsis Dihydroxyacetone (DHA) has been allowed to react with a series of' aminoacids in water solution. The dark solutions or suspensions obtained, were dialysed giving brown 'pigments' (melanoidins) which were subjected to instrumental analysis. Visible absorption spectra of' their water solutions have no special features, while u.v. spectra show at best a slight maximum around 310-330 nm. I.R. spectra of all the products studied show broad bands around 3400, 1600 and 1400 cm-L All these melanoidins exhibit a strong Electron Spin Resonance (E.S.R.) signal. The E.S.R. spectra recorded in the solid state are single lines of 6-12 gauss linewidth, centred around g=2, which correspond to rather inert unpaired electrons since they can be observed in water solution as well. These data suggest that the reaction products of DHA with aminoacids, are conjugated polymers. The mechanism of skin tanning by DHA is discussed in view of these results. Introduction Although the first mention that dihydroxyacetone (DHA) makes human skin brown was published almost 50 years ago (1), still very little is known about the nature of the pig- ments formed. Soon after the first suntan-simulating cosmetic preparations appeared on the market, it was found that aminoacids were able to form brown or black products with DHA (2-4) and it is generally admitted now, that its tanning effect on skin is due to a : ::'.:•-•i' condensation with amino groups of keratin and aminoacids released by sweat. The purpose of the present work was to isolate the ultimate coloured pigments formed in the .reaction of aminoacids with DHA, which are very similar to the melanoidins formed in their so-called MAILLARD reaction with sugars (5), and to carry out a complete i•: i: i: i::' • ..spectroscopic study of these products in order to gain more information about their i structure. •.. ??':: Experimental and results I. REACTIONS CARRIED OUT AT 100øC :i/:?,i. Equimolar quantities (0.1 or 0.05 tool) of DHA and aminoacids usually in 25 ml of water, :ii. •:i! were allowed to react at 100øC on a steam bath for 4 h with refluxing. In some cases ?!ii:?•11 (especially when the aminoacid was not soluble enough) a greater amount of water was ?:'/':?used (50 or 100 ml) and, or, an equimolar quantity of alkali (triethanolamine or sodium :?:•:'! hydroxyde) was added. The reaction started almost immediately with formation of •:/? :•:.::colour: yellow, then red and finally dark brown. In most cases a gas evolution was :i)?! i:' noticeable Some experiments have also been carried out at 37øC using the same quan- ::i•::i ,!:f ?• tities of reactants but for longer periods, usually 24 h. 25
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)