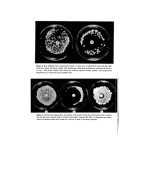
32 A. Meybeck (6) or just a maximum at 327 nm (8), also resemble those of melanoidins. And as far as I.R. is concerned the same bands are found again for both types of pigments since melanins show, as melanoidins do (Table III), a maximum around 3400, and a maximum around 1620 with a shoulder near 1700 cm -x (6, 7, 10, 11). Their percentage compositions are a little different, however, since a typical hair melanin contains 57'1•o C, 3.55/o H, 9'6•o N and 29'4•o O (11) while we found for the (DHA+Gly) melanoidin: 46.75/o C, 5'55/o H and 8-45/o N, the remaining unaccounted for 39'4•o being probably oxygen. All this shows that, although the pigments formed in the reaction of DHA with aminoacids are different by their preparation, they are nevertheless very similar to melanins by their properties and especially by their free spin content. Hypotheses on the structure of melanoidins Still another very interesting result is that E.S.R. spectra of melanoidins can be observed in their aqueous reaction media at room temperature (Fig. 4) showing that the free spin centres, if they were real free radicals, ought to be extremely inert. And it is particularly striking to remark that an unpurified solution of (DHA +Gly) pigment prepared at 37øC and never heated above this temperature, also exhibits a signal. The E.S.R. absorption lines observed in water have the same linewidths (7'5-9.5 G) and apparently the same intensity as the dry powders, although this last point is difficult to certify since a special flat cell of unknown volume had to be used in these experiments due to the high dielectric loss of aqueous solutions. These last results particularly, show that the E.S.R. behaviour of melanoidins is an intrinsic property of their macromolecule and is not due to some kind of free radicals trapped during polymerization. Io G Gly Solutions / • GI Copsule Dial. Und•ol, Figure 4. E.S.R. spectra of: solutions of melanoidins obtained by reacting DHA with glycine for 24 h at 37øC and 6 h at 100øC natural dispersions of melanin pigments in light and dark hair malanoidin obtained by action of DHA on glycine in an open capsule over a steam bath until the reaction medium was dry, and observed in the powder state before and after purification by dialysis. In the following experiment, however, such species may have been formed. DHA (1'8 g) and Gly (1.5 g) were heated with 6 g water in a capsule on a steambath until the brown resultant mass was dry (1 h 30 min). A sample of this unpurified product was kept and
Reaction products of dihydroxyacetone 33 the rest of it was dialysed as usual, with a total yield of 35•o. The E.S.R. spectrum of the purified pigment was similar to that of a melanoidin prepared by refluxing (linewidth H=7 G, spin content=l-8 x 10•S/g. But the signal given by the reaction product before dialysis was quite different (Fig. 4), with a linewidth of 25 G and an intensity correspond- ing to 21 x10 •s spins/g. These striking data can be explained if one assumes that some free radicals were trapped when the reaction medium became viscous by evaporation and finally dried to a vitrous state. These species would then have been lost by reaction with water upon dissolution prior to purification, thus explaining the high loss in free spins. The linewidth of 25 G can also be explained by real free radicals since the spectra of such localized unpaired electrons are likely to exhibit a hyperfine structure (by inter- action with the protons of the molecule) which, although unresolved, would widen the signal significantly. But if, in the general case, purified melanoidins do not contain real free radicals, how can one account for their E.S.R. properties? In recent years, a number of polymers with conjugated double bonds have been found to contain from 10 •ø to 1020 free spins/g (12-15). Their E.S.R. signal is generally a single line of 5-10 G width (12) although a hyperfine structure has been reported in some cases (14, 16) and it can be observed also in solution (12, 16) showing that it must be due to an intrinsic property of the molecule. : This phenomenon has tentatively been explained by several kinds of theoretical con- : siderations (12). It could be due to a semi-conductor effect such as the one proposed to ': account for the paramagnetism of melanins (17, 18). Or it could be, that conjugated polymers in their fundamental electronic state possess, at least partly, the character of :' ':' triplet states (12). Or finally, the free spins observed may be bond alternation defects i:•:•. much like the ones predicted by calculation (19). According to this theory, the alter- nation between double (short) and single (long) bonds along a conjugated polymer chain, i'ii.'::.•i is disrupted by carbon atoms bearing a non-bonding molecular orbital occupied by a :•::•i•: single spin. Such unpaired electrons can travel rapidly through long series of conjugated ß ..i:/,:'double bonds and this deloca!ization is responsible for the lack of hyperfine structure in :•!i:i? the resulting E.S.R. spectra. Two of these defects may be formed through the rupture of one double bond by 5•:11:i.•i: thermal excitation or by lattice deformations (13), or even by photochemical excitation, a "Process which could explain the photoenhancement of melanin E.S.R. signals observed by .:11':ii:'i:iStratton and Pathak (20). In fact the paramagnetic behaviour of melanins should also i•i:::ii::'!be considered as an intrinsic property of their highly conjugated double bonds system, !:•/ and not as a proof of the existence of so-called free radicals in their molecules. In view of these theories, the E.S.R. properties of DHA melanoidins, together with ":•::!'151:i their I.R. absorption around 1600 cm -•, and their dark brown colour due to their non- 'i:!:::,:•: specific absorption in the visible, point out to a highly conjugated double bond structure. monomers of these macromolecules are still unknown, however, although they to be derived from the Schiff base formed by condensation of DHA with amino- ::::.? acids, probably after dehydration and eventually decarboxylation. In the case of the reaction of sugars, it has indeed been shown that the original condensation products lose water very easily to produce furrural derivatives (21-22). Only the alpha •..:!: :i.:Taminoacids are decarboxylated in the process and the non-alpha aminoacid correspond- :•:'i:'.:'.ing pigments exhibit the characteristic absorption band around 1710 cm -• But in any ?:'•??:• case, I.R. spectra show that the rest of the parent aminoacid molecule is always retained • •:•. ':::in DHA melanoidi.n polymers.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)