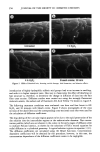
334 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 50 • 40 o Q3 30 '1• 20 I I I 0 10 20 30 40 50 D-Value (Hours) Figure 1. Scatter diagram of the ED-values obtained by the rapid screening method and actual D-values obtained by the linear regression method of 60 cosmetic and OTC-drug samples challenged with different test organisms. value) when the minimum possible ST (MPST) and the APC at time 0 hr are known. It is apparent that products meet preservative system acceptance criteria if the D-value or MPD-value is less than the D-value of the acceptance criteria (i.e., •4 hr for (opportunistic) pathogens, •28 hr for nonpathogens, and bacteriostatic/bactericidal for Bacillus). Use of sufficient concentration of microorganisms in the inoculum (i.e., 106 organ- isms/g in the sample) and sampling at the time to determine the ST or MPST provide sufficient information to determine whether the product meets the acceptance criteria for specific test organisms when using the rapid screening method. The ED-values provided by the rapid screening method are based on MPST and should meet acceptance criteria of the linear regression method. In addition to use of APCs at time 0 and at later times, ED-values have been calculated for scores of samples using a "virtual" survivor curve (6). A virtual survivor curve is made using the APC of the inoculum for each test organism to calculate the initial APC, as is done by the USP method (1), and at other times to obtain ED-values to meet acceptance criteria (i.e., APCs at 24 hr for pathogens and at 7 d for nonpathogenic bacteria, yeasts, and molds). The estimation of D-values using two APCs provides substantial time and material savings over the original linear regression method because a number of samples can be inoculated without the requirement for determining APCs of each test organism in each sample immediately after inoculation and at intermediate time points.
PRESERVATIVE EFFICACY TESTING 335 ED-values may be determined using saline suspensions of pure cultures or mixed inocula (i.e., where more than one test organism was used in the saline suspension added to a test sample). We have found that use of pooled inocula is convenient when using organisms with similar ED-values (i. e., P. aeruginosa and S. aureus E. coli and P. cepacia) and/or recovery media (i.e., C. albicans and A. niger). Microbiologists generally use duplicate plates for each dilution when determining APCs. This is done to improve the reliability of the plating method and to help overcome human factors, which decrease the precision of the method (i.e., inaccuracies in pipeting). Preliminary data in our laboratory indicate that use of single plates and duplicate plates give essentially the same D-values. The difference in D-values was 0.5 hr for those tests in which the maximum D-values were no larger than 12 hr. Although the differences in D-values may be greater with larger D-values, it is believed that the percentage difference would be 10% as long as the assays are "in control" (7-9). Laboratories should implement statistical control procedures to ensure that assays are in control. Our preliminary data suggest that in some instances a laboratory may be able to use single plates for rapid screening studies. This work describes a rapid screening method for estimating D-values. This method is similar to the linear regression method however, intermediate samplings and APCs are omitted. This allows determination of an ED-value using APCs at 0 hr and 24 hr (for pathogens) and at 0 hr and 7 d (for non-pathogenic bacteria, yeast, and molds). When using an inoculum of 10 6 organisms/g product, recovery of 10 organisms/g product at 24 hr or 7 d indicates that the MPST is 424 hr or 4 168 hr, respectively, and that the ED-value is 44 hr or 428 hr, respectively. The reliability of the rapid screening method was good over the range of D-values one finds in both satisfactorily and unsatisfactorily preserved products (i.e., D-values 4 hr to 39 hr). There was excellent agreement between ED-values and D-values (correlation coefficient = 0.98), and the difference between mean D-values and mean ED-values was well below 10%. This is considered to be suitable for a rapid screening method. Where differences in estimated and D-values were observed, the ED-values generally were larger (i.e., more conservative) than D-values for the same samples. The physicochemical make-up of each formula, the type of packaging, and conditions of use by consumers determine the risk of microbial contamination and spoilage (10). Several formulas frequently must be tested in the process of selecting the final formula. The rapid screening method provides a convenient means of selecting the preservative system of a product and offers about 50% savings in terms of labor and materials required for testing. It is recommended that ED-values be confirmed by determining D-values for finished formulations. ACKNOWLEDGMENT We thank Ms. Fariba Jashnian for her assistance with the microbiological analyses. REFERENCES (1) Microbiological Tests, Antimicrobial Preservatives--Effectiveness, United States Pharmacopeia XXII, United States Pharmacopeial Convention, Rockville, MD, pp. 1478-1479 (1990).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)