336 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) Efficacy of antimicrobial preservatives in pharmaceutical products, British Pharmacopoeia 1988, Vol. II., Her Majesty's Stationery Office, London, England, pp. A200-A208 (1988). (3) Preservation Subcommittee of the CTFA Microbiological Committee, A guideline for the determi- nation of adequacy of preservation of cosmetics and toiletry formulations, TGA Cosmet. J., 2, 20-23 (1970). (4) D. S. Orth, Linear regression method for rapid determination of cosmetic preservative efficacy., J. Soc. Cosmet. Chem., 30, 321-332 (1979). (5) D. S. Orth, Standardizing preservative efficacy test data, Cosmet. Toiletr., 106(3), 45-48,51 (1991). (6) D. S. Orth, C. M. Lutes Anderson, D. K. Smith, and S. R. Milstein, Synergism of preservative system components: Use of the survival curve slope method to demonstrate anti-Pseudomonas synergy of methyl paraben and acrylic acid homopolymer/copolymers in vitro, J. Soc. Cosmet. Chem., 40, 347-365 (1989). (7) D. S. Orth and L. R. Brueggen, Preservative efficacy of cosmetic products. Rechallenge testing and reliability of the linear regression method, Cosmet. Toiletr., 97(5), 61-65 (1982). (8) D. S. Orth, "Principles of Preservation," in S. P. Denyet and R. M. Baird, Eds., Guide to Microbi- ological Control in Pharmaceuticals (Ellis Horwood, London, 1990), pp. 241-250. (9) D. S. Orth, Preservative efficacy testing: Rationale, acceptance criteria, and determination of syner- gism. Oral presentation at The First Pan European Conference on the Significance and Validation of Test Methods for the Efficacy of Preservatives in Cosmetics, Toiletries and Pharmaceuticals, held November 20-21, 1991, at the Commonwealth Institute, London, England. (10) D. S. Orth, The required D-value: Evaluating product preservation in relation to packaging and consumer use/abuse. Cosmet. Toiletr., 107, 39-43 (1992).
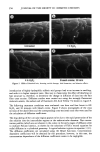
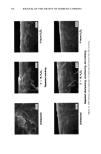
j. Soc. Cosmet. Chem., 44, 337-345 (November/December 1993) Consistency development and destabilization of a model cream LORRAINE E. PENA, BARBARA L. LEE, and JAMES F. STEARNS, Drug Delivery R&D--Specialty Products, The Upjohn Company, Kalamazoo, MI 49007. Received.July 22, 1992. Presented at the 16th IFSCC Congress, New York, October 1990. Synopsis Emulsions are thermodynamically unstable and with time show progressive signs of this instability with eventual phase separation. It is also an established fact that creams based on nonionic emulsifier systems exhibit an initial period of delayed consistency development prior to the destabilization process. To illustrate these phenomena, a cream having a nonionic emulsifier system is destabilized by a surface-active ingredient that also exaggerates the period of retarded consistency development. Using rheologic and microscopic techniques, this study presents a systematic approach by which consistency development and destabilization can be monitored. Characteristic patterns in the rheograms upon aging are correlated with changes seen microscopically, and specific changes signaling the beginning of the destabilization process are identified. Rheologically, destabilization becomes apparent through formation of additional spurs and inflections at low shear rates, a decrease in hysteresis, and a shift to lower maximum shear stress values. Microscopically, polarized light shows formation of diffuse, weakly birefringent structures while ordinary light shows an increase in droplet size. Thermal optical videomicroscopy and trace substance analysis have identified the structures as segments of agglomerated oil phase and verify the photomicroscopy observation that the oil and wax components phase separate as distinct entities. INTRODUCTION All emulsion systems undergo a process of alestabilization and phase separation. How- ever, creams based on nonionic emulsifier systems first undergo an initial period in which their consistency slowly develops. The gel network theory has shown that the consistency of a cream is due to structuring of the continuous phase via penetration and swelling of the fatty amphiphile component by the aqueous surfactant phase to form a viscoelastic, lamellar liquid crystalline gel network (1-6). In the case of nonionic surfactants, this penetration is delayed, and therefore consistency development is also delayed (3). This paper presents a systematic approach by which consistency develop- ment and alestabilization can be monitored. A cetearyl alcohol/ceteareth-20 cream is alestabilized by the addition of a surface-active ingredient that also exaggerates the period of delayed consistency development. Rhe- ology and microscopy are used to monitor consistency development and alestabilization 337
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)