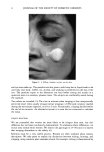
10 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS on a wash-out period before assessing subjective and objective reactions to potentially irritating materials. TACHYPHYLAXIS? This esoteric term applies to a well known phenomenon in which a predicted response to a chemical fails to occur upon repeated challenge. For example, the vasoconstriction induced by a six-hour exposure to a corticosteroid will not occur after three to four such exposures daily. The site becomes refractory. We were cautious not to repeat the lactic acid test too frequently on the same site for fear of intensifying the stinging response. Previous reports have warned against repeated exposures in order to prevent intensified reactions (9). However, reexposures every other day or every week on the same site did not, as we expected, augment the response. The scores either remained nearly the same or the reactions actually diminished, suggesting tachyphylaxis. More work is needed to define the dimensions of this phenomenon. How tachyphylaxis occurs is unknown. Perhaps receptors become saturated, the horny layer becomes less permeable, or follicular shunts become blocked in some way. Desensitization to the sensations induced by repeated exposures to capsaicin is well known (4). CONCLUSIONS The ability to identify stingers and classify them into mild, moderate, and severe opens up new clinical applications. There is tentative evidence that stingers are more likely to have "sensitive" skin. Muizzuddin et al. found that women who reported higher intol- erance to various products had lactic acid stinging scores that were more than three times that of persons without "sensitive" skin (13). These same individuals were also much more likely to develop irritation after exposure to Balsam of Peru. It would seem worthwhile to test new products on stingers prior to marketing. Our practice is to apply finished formulations twice daily for two weeks to the entire face of moderate stingers. Lactic acid stinging is scored at baseline and one to two days after two weeks of product usage. An appreciable increase in stinging scores would signal a potential for the occurrence of disagreeable reactions in users who have "sensitive" skin. On the other hand, a reduction in stinging scores would indicate a beneficial, protective effect. We have witnessed both outcomes in preliminary trials of proprietary "moisturizers." The stinging phenomenon is still mysterious. We are ignorant of the neurophysiologic mechanisms that underlie it. Why some apparently normal women sting severely and others not at all is a question worthy of serious investigation. Stingers cannot be recognized by phenotype or by clinical examination. Many factors are undoubtedly at play and can only be brought to light by focused research. Among the variables that might affect stinging are age, ethnicity, sex, atopic background, barrier function, cosmetic practices, and degree of oiliness or dryness. We are looking into all of these. A huge variety of skin care products and cosmetics have been designed for facial application. The predictive value of the stinging test must take into account the in- tended use of the product. For example, Grove et al. determined that a commercial formulation did not cause adverse effects in a panel of stingers (9). Nonetheless, the
LACTIC ACID STINGING TESTS 11 manufacturer had to recall the product after marketing because of disagreeable reactions. It was then learned that the product was designed for the eye area. It is well known that the thinner eyelids are more susceptible to irritation. Eye area products should therefore be tested directly on that area. Products designed for a specific area of the face should be tested directly on that area. REFERENCES (1) P. J. Frosch and A. M. Kligman, A method for appraising the stinging capacity of topically applied substances, J. Soc. Cosmet. Chem., 28, 197 (1977). (2) A. Fisher, Cosmetic actions and reactions: Therapeutic, irritant and allergic, Cutis, 26, 22 (1980). (3) A. W. Johnson and D. J. Page, Making sense of sensitive skin. Presented at the IFSCC, Yokohama (poster), 1992. (4) B. G. Green and B. S. Bluth, Measuring the chemosensory irritability of human skin,J. Toxicol. Cut. Ocul. Toxicol. 14, 230 (1995). (5) P. J. Frosch and A.M. Kligman, "Recognition of Chemically Vulnerable and Delicate Skin," in Principles of Cosmetics for the Dermatologist, P. J. Frost, Ed. (Mosby, St. Louis, 1981). (6) D. Soschin and A. M. Kligman, "Adverse Subjective Responses," in Safety & Efficacy of Topical Drugs and Cosmetics, A. M. Kligman and J. J. Leyden, Eds. (Grune & Stratton, New York, 1982). (7) D. R. Armstrong, M. L. Dry, C. A. Keele, and J. W. Markham, Methods for studying chemical excitants of pain in man, J. Physiol, 115, 59 (1951). (8) K. Lammintausta, H. I. Maibach, and D. Wilson, Mechanisms of subjective irritation, Dermatosen, 36, 45 (1988). (9) G. Grove, D. Soschin, and A. M. Kligman, Guidelines for performing facial stinging tests, Proc. 12th Congress Internat. Fed. Soc. of Cosmet. Chem, Paris, September 13-17, 1982. (10) J. R. Mayne, O. H. Mills, and J. C. Lyssikatos, Lactic acid sting assay: Reproducibility and sym- metry of subjective sting response. J. Derm. C/in. Eva/. Soc., 3, 63 (1992). (11) P. J. Frosch, "Cutaneous Irritation," in Textbook of Contact Dermatitis, R. J. G. Rycroft, T. Menne, and P. J. Frosch, Eds. (Spring-Verlag, New York, 1995), pp. 28-61. (12) A. Pagnoni, A. M. Kligman, S. el Gainreal, C. Popp, and T. Stoudemayer, An improved procedure for quantitative analysis of sebum production using Sebutape, J. Soc. Cosmet. Chem, 45, 221 (1994). (13) N. Muizzuddin, K. D. Marenus, C. Ethenakis, and D. Maes, Skin reactivity and barrier condition. Presented at the American Academy of Dermatology, Washington, DC, December 4-9, 1993.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)