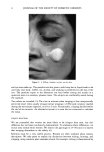
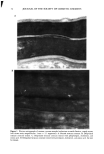
j. Soc. Cosmet. Chem., 47, 13-26 (January/February 1996) Mechanism of tensile stress release in the keratin fiber cuticle: I SIGRID B. RUETSCH and HANS-DIETRICH WEIGMANN, TRI/Princeton, PO Box 625, Princeton NJ 08542. Received September 15, 1995. Synopsis During the extension of keratin fibers, their two major morphological components, the cuticula and the cortex, accommodate the stresses imposed on the fiber each in a totally different fashion. While the latter extends by mechanisms that have been discussed extensively and appear to be well understood, the cuticle cells are essentially inextensible and have to move relative to one another. In the multilayer structure of the cuticular sheath of human hair fibers, this relative movement has to be accommodated by the various layers within each cuticle cell and by the bonding layers between cells, and finally causes the lifting of surface scale edges at higher strain levels. It is proposed that extension mainly causes shear stresses between layers of different composition and extensibility within the cuticle cell. This leads to failure in the weak endocu- ticular layer and results in "delamination" and lifting of the outer layers of the surface cuticle. The damage is irreversible upon release of the fiber and immersion in water, as reflected in the onset of scale lifting at considerably lower strain levels during a second extension. Scale lifting was not observed during the extension of wool fibers, which appears to be a reflection of the higher rigidity of the cuticle cells of wool. INTRODUCTION Standard grooming practices, such as shampooing, combing, and brushing, can cause considerable damage to the cuticle of human hair, as summarized recently by C. R. Robbins (1). Apart from the general abrasive loss of cuticle layers, which can be quite severe and can eventually lead to the complete loss of the cuticular sheath and formation of split ends (2-5), there are various processes that damage the cuticular structure in such a way that scale edges become particularly vulnerable to abrasive action. One of the processes of damage introduction is the imposition of stresses on individual hair fibers during combing, especially when encountering a snag. These stresses result in reversible fiber extension as well as in some irreversible processes involving the cuticle. The two major morphological components of the hair fiber respond quite differently to the stresses introduced into the fiber during extensions beyond the yield point. The cortex is able to release stresses by the unfolding of o• helical structures into the pleated sheet arrangement of the [3 structure. This o•-[3 transformation occurs in the microfibrils that are embedded in and interconnected to the disulfide cross-linked noncrystalline matrix material that deforms along with the unfolding of the o• helices. There is thought 13
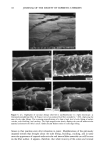
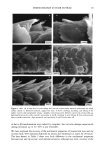
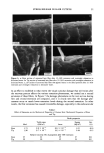
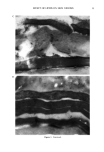
14 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS to be no relative displacement of cortical cells. This mechanism of deformation, which is reflected in the stress-strain curve of the fiber, has been discussed extensively in the literature and appears to be well understood and generally accepted. The deformation response of the other major morphological component, the cuticular sheath, is much less well understood. In torsional deformation, Wolfram and Albrecht (6) have found that in the wet state the modulus of the cortex is twenty times greater than that of the cuticula, while in the dry state the torsional properties of cortex and cuticula are similar. In extensional deformation, on the other hand, it is thought that the cuticula does not contribute to the stress-strain curve of the fiber. The cuticle cells are much more rigid in the longitudinal direction and do not have the capacity to release stress by transformation on a molecular level like the cortical cells. Since the cuticular sheath has to deform concomitantly with the deformation of the cortex and the whole fiber, it is assumed that the deformational stress that occurs on the cuticular sheath is released by the movement of cuticle cells relative to each other. The individual cuticle cell is a multilayered structure enveloped by a cellular membrane, the epicuticle, and it adheres to neighboring cuticle cells through the intercellular cement. The outermost cuticle layer is the highly disulfide cross-linked A layer in which 30-35% of the amino acids are half cystine, making it extremely tough and inexten- sible. The next layer, the exocuticle, has a somewhat lower cross-link density and would be expected to be somewhat more easily deformable. The innermost layer is comprised of the much less cross-linked, easily swellable, and highly extensible endocuticle. Dur- ing extension the difference in deformability of the various layers would be expected to result in the generation of shear forces that develop between these layers. At higher extension levels these shear forces can lead to stress concentrations and failure in the weakest layer, the endocuticle. We have studied the response of the surface cuticle of human hair and wool fibers to extension by fluorescence and scanning electron microscopy and will discuss details of that investigation in the following paragraphs. While extension levels used in this investigation are mostly higher than those normally encountered in hair grooming procedures, our results shed light on the deformation processes in the cuticular sheath and its most vulnerable components. EXPERIMENTAL MICROFLUOROMETRY Individual hair fibers are mounted in an extension frame and extended manually under ambient conditions (-50% relative humidity, room temperature) while one observes the fiber in a fluorescence microscope (Leitz MPV 1.1 with Ploemopak attachment). Upon excitation in the ultraviolet range (nonpolarized light, 340-380 nm), the un- treated unextended hair fiber tends to glow with a weak and diffuse blue-white autof- luorescence (Figure 1, left). This intrinsic fluorescence of proteins upon excitation in the UV is based on the presence of certain aromatic amino acids, i.e. tryptophane, tyrosine, and phenylalanine (7,8). It is known that the disulfide groups of cystine are fluorescence quenchers and that their environmental or oxidative scission leads to a significant increase in fluorescence intensity. During extension the surface cuticle edges become
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)