156 JOURNAL OF COSMETIC SCIENCE range of cosmetic effects, such as skin smoothening, moisturizing, prevention of pre- mature skin-aging, suppression of UV-induced erythema, or improved wound healing (4). Vitamin E is readily oxidized when exposed to atmospheric conditions or light (5). In consequence, free vitamin E is only rarely used in cosmetic formulations. It is replaced by stable esterified pro-drugs, usually vitamin E acetate (6). However, such esters are biologically inactive. If applied topically, vitamin E acetate has been reported to be hydrolyzed in the skin to free vitamin E by esterases (7-9). Yet, despite the many reports documenting their biological activity and implying a conversion of the pro-drug, direct quantitative evidence for a conversion of vitamin E acetate to vitamin E in skin is poor and ambiguous (10,11). Vitamin E acetate and vitamin E are both lipophilic compounds. They dissolve in the oil phase of a formula, from where they are expected to be poorly liberated. To increase bio-availability they are commonly formulated in the water phase of a cosmetic formula by means of solubilizers or by specific carriers (6). In the present study, we compared the penetration behavior of vitamin E acetate under non-occlusive and occlusive conditions. In turn, the pro-drug was either dissolved in oil, solubilized in water, or encapsulated in two different carrier systems, i.e., liposomes or Nanotopes TM, the latter being detergent-resistant unilamellar vesicles with a diameter of 20-40 nm (12). The amount of both vitamin E acetate and vitamin E was quantified in three compartments: at the skin surface, in the horny layers, and within the underlying skin. MATERIALS AND METHODS SKIN BIOPSIES Human skin was obtained after informed consent from otherwise healthy donors having undergone plastic surgery for stomach reduction. Upon excision, the skin flaps (ellip- soid, 5 x 20 cm) were surgically liberated of the adhering subcutaneous fat layer, cut to the size of the penetration chamber, and immediately stored in PBS (phosphate-buffered saline, pH 7.2-7.4) at 36øC. TEST FORMULATIONS Final concentrations of 2% vitamin E acetate were dissolved in Mygliol-812N (caprylic/ capric acid triglyceride Condea Chemie GmbH, [Brunsbiittel, Germany]), solubilized in water by Solubilisant-LRI (PPG-26-buteth-26 and PEG-40 hydrogenated castor oil LCW-Wackherr [Saint Ouen L'Aum6ne, France]), encapsulated in liposomes made from soybean phosphatidyl choline with an average mean diameter of 200 nm (Mica Products GmbH [Badenweiler, Germany]), or encapsulated in Nanotopes TM with a mean diam- eter of about 25 nm (Tinoderm E, Ciba Specialty Chemicals [Basel, Switzerland]). PENETRATION Skin flaps were mounted within 1 h after excision in Franz-like perfusion chambers according to Maibach (13), which had been modified as described (14). The skin sup-
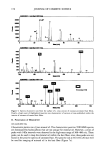
CONVERSION OF VITAMIN E ACETATE TO VITAMIN E 157 ported by a grid was fitted in the permeation chamber (surface area 28 cm2). Its underside was in contact with degassed PBS, avoiding air bubble formation. The cham- ber was covered with a bell-shaped lid equipped with cocks and enclosing a volume of 46 cm 3, thus permitting the maintenance of controlled conditions during permeation experiments, e.g., to mimic open or occlusion-like conditions. The maintenance of enzymatic activity in the excised skin was monitored as described (13,14). Test solutions of 300 pl were applied to the surface of the mounted skin and evenly spread with a glass spatula. Experiments were carried out under controlled conditions at 23øC and at a maintained humidity of 40%. All experiments were carried out in triplicate and with the skin from three different donors. Experiments were rejected if vitamin Etota 1 was detected in the PBS media ( 100 ng/ml) within 10 min after appli- cation. ASSESSMENT OF VITAMIN E DISTRIBUTION IN SKIN The distribution of both vitamin E acetate and vitamin E was monitored 8 h after application. Vitamin Etota • was collected from the skin surface by wiping with a cotton sponge (Ix dry, lx with 200 pl methanol, l x dry). The horny layer was essentially removed by 20 strippings using adhesive tape (Tesa-Film © [Beiersdorf, Hamburg, Ger- many]), and the remaining skin was frozen and sectioned. Cotton sponges, tape strips, and skin sections were extracted with methanol/phosphate buffer (50/50, v/v). QUANTIFICATION OF VITAMIN E ACETATE AND VITAMIN E BY HIGH-PRESSURE LIQUID CHROMATOGRAPHY (HPLC) Concentrated extracts were separated by HPLC using a reverse-phase column (Luna C 18, 5 pm, 150-mm length, 4.6 mm O, Penomenex © [Aschaffenburg, Germany]) and a mobile phase of methanol/acetonitrile (25/75, v/v). Injection volumes were 100 or 250 pl, and the sensitivity of detection was 25-50 ng/ml. RESULTS Four formulations, each containing 2% vitamin E acetate, i.e., dissolved in Mygliol- 812N (EM), solubilized in water (ES), or encapsulated in liposomes (EL) or in Nano- topes TM (ET), were tested. Of each formulation 11 mg/cm-' was applied to the skin, corresponding to 220 pg/cm 2 of vitamin E acetate. The skin was then maintained for 8 h under defined non-occlusive or occlusive conditions, respectively. Then the amount of vitamin E acetate and vitamin E (tocopherol) was determined for the three compart- ments (skin surface, horny layer, underlying skin). In all experiments, the recovery rate of vitamin Etota • (vitamin E acetate + vitamin E) exceeded 90%. The vitamin E acetate preparations contained --2% of vitamin E prior to application. DISTRIBUTION OF VITAMIN EToT^ r UNDER NON-OCCLUSIVE CONDITIONS Under non-occlusive conditions and when applied in the oil phase (EM), 178.3 (+8%, SD = 3) lng/cm 2 vitamin Eto• l was detected on the skin surface and 28.2 (+31%) lng/cm 2
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)