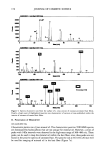
160 JOURNAL OF COSMETIC SCIENCE 100' + 80 m 60 ._c.• 40 2O EM ES EL ET vitamin E acetate ':• free vitamin E Figure 2. Influence of formulation on distribution of vitamin E acetate and vitamin E in human skin. Application on excised human skin under air occlusive conditions. Vitamin E acetate was dissolved in Mygliol (EM), solubilized in water (ES), and encapsulated in liposomes (EL) and in Nanotopes TM (ET). under occlusive conditions was smaller, conversion amounted to about 50% in each of the three formulations (see Table I). DISCUSSION Vitamin E is known as nature's major lipid-soluble free-radical scavenging antioxidant (4). Due to its instability in the presence of oxygen or light, the use of free vitamin E as a cosmetic active is very limited. Therefore, it has been replaced by stable, oil-soluble precursors, i.e., mainly vitamin E acetate or palmitate. These derivatives are claimed to be converted in the skin to the active vitamin E (4,5). The present study corroborated that vitamin E acetate is a suitable precursor for vitamin E in topical applications. Quantitative analysis demonstrated that vitamin E acetate was only available in the living skin if formulated into a water phase and not in an oil phase. Lipophilic substances are incorporated into the water phase either by means of solubi- lizers or with carrier systems such as liposomes or Nanotopes TM. In the present study, Nanotopes TM deposited more vitamin E acetate in skin than liposomes or the solubilizer under both non-occlusive and occlusive conditions. This consistently better performance of ET may relate to the size of Nanotopes TM, which is about ten times smaller than that of conventional liposomes (12). Bioconversion of vitamin E acetate to vitamin E was localized exclusively in the viable skin (most probably in the epidermal layer [Artmann, unpublished]). Hydrolysis was
CONVERSION OF VITAMIN E ACETATE TO VITAMIN E 161 absent on the skin surface as well as in the horny layer. These findings are in accordance with Landmann (15), who reported extracellular esterases being shed by lameliar bodies in the epidermis. As demonstrated, the galenic formulation of the vitamin E acetate, as of lipophilic substances in general, is crucial for its skin availability. Beyond the physical and chemi- cal properties of the active, the type of formulation and the phase in which the active is dissolved also determine its fate and the biological activity. In the present study, more than 95% of vitamin E acetate remained either on the skin surface or deposited in the horny layer when applied in an oil. In contrast, when applied in the water phase, up to 50% of vitamin Etot• l was made available to the viable skin, the zone of cosmetic interest. In the best case, up to 20% of the initial amount of vitamin E acetate was recovered as free vitamin E. Controversial findings on the activation of the pro-drug may well be due to differences in formulating vitamin E acetate (7-11). REFERENCES (1) A. Igarashi, M. Uzuka, and K. Nakajima, The effects of vitamin E deficiency on rat skin, Br. J. Dermatol., 121, 43•49 (1989). (2) J. Fuchs, M. Huflejt, L. M. Rothfuss, D. S. Wilson, G. Carcamo, and L. Packer, Acute effects of near ultraviolet and visible light on the cutaneous antioxidant defense system, Photochem. Photobiol., 50, 739-744 (1989). (3) M.M. Rieger, Oxidative reactions in and on skin: Mechanism and prevention, Cosmet. Toiletr., 108, 43-55 (1993). (4) P. Mayer and W. Pittermann, The effects of vitamin E on the skin, Cosmet. Toiletr., 108, 99-109 (1993). (5) V. Kagan, E. Witt, R. Goldmann, G. Scita, and L. Packer, Ultraviolet light-induced generation of vitamin E radicals and their recycling. A possible photosensitizing effect of vitamin E in the skin, Free Rad. Res. Comm., 16, 51-64 (1991). (6) M. Rangarajan and J. Zatz, Skin delivery of vitamin E,.]. Cosmet. Sci., 50, 249-279 (1999). (7) E. P. Norkus, G. F. Bryce, and H. N. Bhagavan, Uptake and bioconversion ofalpha-tocopheryl acetate to alpha-tocopherol in skin of hairless mice, Photochem. Photobiol., 57, 613-615 (1993). (8) K. Tojo and A.-R. C. Lee, Bioconversion of a provitamin to vitamins C and E in skin, J. Soc. Costa. Chem., 38, 333-339 (1987). (9) J. R. Trevithick and K. P. Mitton, Topical application and uptake of vitamin E acetate by the skin and conversion to free vitamin E, Blochem Mol. Biol. Intl., 31,869-878 (1993). (10) H. Gensler, M. Aikin, Y-M. Peng, and M. Xu, Importance of the form of topical vitamin E for prevention of photocarcinogenesis, Nutr. Cancer, 26, 183-191 (1996). (ll) D. S. Alberts, R. Goldmann, M.-J. Xu, R. T. Dorr, J. Quinn, K. Welch, J. Guillen-Rodriguez, M. Aickin, Y.-M. Peng, L. Loescher, and H. Gensler, Disposition and metabolism of topically adminis- tered alpha-tocopherol acetate: A common ingredient of commercially available sunscreens and cos- metics, Nutr. Cancer, 26, 193-201 (1996). (12) B. Herzog, K. Soreruer, W. Baschong, and J. Roeding, Nanotopes, a surfactant resistant carrier system, SOeFWJournal, 124, 614-623 (1998). (13) H. Maibach, Percutaneous absorption,.]. Am. Coll. Toxicol., 8, 803-813 (1989). (14) C.W. Artmann, In vitro percutaneous absorption into human skin, Fund. Appl. Toxicol., 28, 1-5 (1996). (15) L. Landmann, The epidermal permeability barrier, Anat. Emb•yol., 178, 1-13 (1988).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)