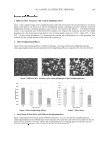
JOURNAL OF COSMETIC SCIENCE 340 total anthocyanins) was added to 100 g of the cosmetic cream, the UV absorption ability of the cosmetic cream was signifi cantly improved, reaching 46%. When 10 ml of the antho- cyanin extract (1.22 mg of total anthocyanins) was added, 50% of the UV radiation was absorbed. Based on Figure 5, anthocyanin extract contributed to the absorption of UV ra- diation. However, the relationship between UV absorption and anthocyanin extract content was not linear. The improvement in UV absorption ability became less signifi cant as more anthocyanin extract was added. The optimal anthocyanin extract content is dependent on practical application and requires further study. Generally speaking, a small amount of an- thocyanin extract was adequate for signifi cant enhancement of UV absorbing ability. The recipe provided in this study was designed to show the effect of adding purple sweet po- tato extract to a cosmetic cream. The UV-absorbing ability of anthocyanin extracts was mea- sured by using a UV spectrophotometer without running expensive human tests. Although the UV spectrophotometric methodology is a convenient tool for preliminary evaluation, hu- man tests are still required to confi rm the infl uence of anthocyanins on humans. The next step of this study is to get governmental permission for running SPF analysis on humans. CONCLUSIONS TNG73 purple sweet potato extracts were an excellent anthocyanin source for preparing UV-protective cosmetic cream. The acidic ethanol-extracted anthocyanins had better radical scavenging ability, higher total phenolic content, and stronger reducing ability than anthocyanins extracted using acidic water. Based on these results, we recommended the use of acidic ethanol-extracted anthocyanins as additives in UV-protective cosmetic cream. The UV absorption ability of the cosmetic cream was successfully improved by the addition of anthocyanin extract. The difference in UV absorption ability arising from the addition of anthocyanin extract was more signifi cant for UV-B rays. However, the opti- mal anthocyanin extract content remains dependent on practical application, and requires further study. ACKNOWLEDGMENTS This research was funded by Chang Gung Institution of Technology, grant no. EZRPF380141. REFERENCES (1) H. Hidaka, S. Horikoshi, N. Serpone, and J. Knowland, In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation, J. Photochem. Photobiol. A: Chem., 111, 205–213 (1997). (2) A. Ouhtit and H. N. Ananthaswamy, A model for UV-induction of skin cancer, J. Biomed. Biotech., 1, 5–6 (2001). (3) H. Soehnge, A. Ouhtit, and H. N. Ananthaswamy, Mechanisms of induction of skin cancer by UV ra- diation, Frontiers in Biosci., 2, 538–551 (1997). (4) D. Moyal, How to measure UVA protection afforded by sunscreen products. Expert Rev. Dermatol., 3, 307–313 (2008). (5) N. Serpone, A. Salinaro, S. Horikoshi, and H. Hidaka, Benefi cial effects of photo-inactive titanium di- oxide specimens on plasmid DNA, human cells and yeast cells exposed to UVA/UVB simulated sun- light, J. Photochem. Photobiol. A: Chem., 179, 200–212 (2006).
PURPLE SWEET POTATO EXTRACTS IN UV PROTECTION 341 (6) S. A. Wissing and R. H. Müller, Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm., 254, 65–68 (2003). (7) N. A. Shaath, “Evolution of Modern Sunscreen Chemicals,” in Sunscreens—Development, Evaluation, Regu- latory Aspects, 2nd ed., N.J. Lowe, N.A. Shaath, and M.A. Pathak, Eds. (Marcel Dekker, New York, 1997), pp. 3–34. (8) N. A. Shaath, “The Chemistry of Sunscreens,” in Sunscreens—Development, Evaluation, Regulatory Aspects 2nd ed., N.J. Lowe, N.A. Shaath, and M.A. Pathak, Eds. (Marcel Dekker, New York, 1997), pp. 263–284. (9) U. Heinrich, H. Tronnier, D. Kockott, R. Kuckuk, and H. M. Heise, Comparison of sun protection factors determined by an in vivo and different in vitro methodologies: A study with 58 different com- mercially available sunscreen products, Int. J. Cosmet. Sci., 26, 79–89 (2004). (10) M. F. Bobin, M. Raymound, and M. C. Martini, UVA/UVB absorption properties of natural products, Cosmet. Toiletr., 109, 63–70 (1994). (11) T. Hampton, Broccoli extract may help reduce UV skin damage, JAMA, 298, 2731–2736 (2007). (12) A. Svobodová, J. Psotová, and D. Walterová, Natural phenolics in the prevention of UV-induced skin damage: A review, Biomed. Papers, 147, 137–145 (2003). (13) A. Torres, C.D. Enk, M. Hochberg, and M. Srebnik, Porphyra-334, a potential natural source for UVA protective sunscreens, Photochem. Photobiol. Sci., 5, 432– 435 (2006). (14) X. Duan, Y. Jiang, X. Su, Z. Zhang, and J. Shi, Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning, Food Chem., 101, 1365–1371 (2007). (15) K. H. Han, M. Sekikawa, K. I. Shimada, M. Hashimoto, N. Hashimoto, T. Noda, H. Tanaka, and M. Fukushima, Anthocyanin-rich purple potato fl ake extract has antioxidant capacity and improves anti- oxidant potential in rats, Br. J. Nutr., 96, 1125–1133 (2006). (16) M. Kano, T. Takayanagi, K. Harada, K. Makino, and F. Ishikawa, Antioxidative activity of anthocyanins from pruple sweet potato, Ipomoera batatas cultivar ayamurasaki, Biosci. Biotechnol. Biochem., 69, 979–988 (2005). (17) F. Cimino, R. Ambra, R. Canali, A. Saija, and F. Virgili, Effect of cyanidin-3-O-glucoside on UVB- induced response in human keratinocytes, J. Agric. Food Chem., 54, 4041–4047 (2006). (18) N. Nakajima, M. Sugimoto, H. Yokoi, H. Tsuji, and K. Ishihara, Comparison of acylated plant pig- ments: Light-resistance and radical-scavenging ability, Biosci. Biotechnol. Biochem., 67, 1828–1831 (2003). (19) A. Tsukui, A. Suzuki, S. Nagayama, and N. Terahara, Stability of anthocyanins pigments from purple leaves of Perilla ocimoides L. var. crispa, Nippon Shokuhin Kagaku Kogaku Kaishi, 43, 451–457 (1996). (20) K. Torskangerpoll and O.M. Andersen, Colour stability of anthocyanins in aqueous solutions at various pH values, Food Chem., 89, 427–440 (2005). (21) A. Kirca, M. Ozkan, and B. Cemeroglu, Effects of temperature, solid content and pH on the stability of black carrot anthocyanins, Food Chem., 101, 212–218 (2007). (22) L. Gao and G. Mazza, Extraction of anthocyanin pigments from purple sunfl ower hulls, J. Food Sci., 61, 600–603 (1996). (23) Z. Y. Ju and L.R. Howard, Effects of solvent and temperature on pressurized liquid extraction of antho- cyanins and total phenolics from dried grape skin, J. Agric. Food Chem., 51, 5207–5213 (2003). (24) L. Longo and G. Vasapollo, Extraction and identifi cation of anthocyanins from Smilax aspera L. berries, Food Chem., 94, 226–231 (2006). (25) E. Revilla, J. Ryan, and G. Martin-Ortega, Comparison of several procedures used for the extraction of anthocyanins from red grapes, J. Agric. Food Chem., 46, 4592– 4597 (1998). (26) A. Tsukui, Stability of anthocyanins in various vegetables and fruits, Food Sci. Technol. Int., 2, 30–33 (1996). (27) M. M. Giusti and R. E. Wrolstad, “Anthocyanins: Characterization and measurement with UV-Visible Spectroscopy,” in Current Protocols in Food Analytical Chemistry. R. E. Wrolstad, Ed. (John Wiley & Sons, Inc., New York, 2000), pp. F.1.2.1–F.1.2.13. (28) Y. C. Huang, Y. H. Chang, and Y. Y. Shao, Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan, Food Chem., 98, 529–538 (2006). (29) T. S. Kujala, J. M. Loponen, K. D. Klika, and K. Pihlaja, Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three indi- vidual compounds, J. Agr. Food Chem., 48, 5338–5342 (2000). (30) N. Singh and P. S. Rajini, Free radical scavenging activity of an aqueous extract of potato peel, Food Chem., 85, 611–616 (2004).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)